Novel 3-Amino-6-chloro-7-(azol-2 or 5-yl)-1,1-dioxo-1,4,2-benzodithiazine Derivatives with Anticancer Activity: Synthesis and QSAR Study
Abstract
:1. Introduction

2. Results
2.1. Chemistry


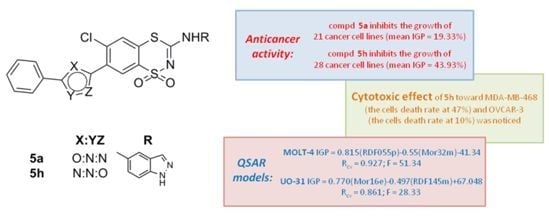
2.2. Anticancer Activity
2.3. QSAR Studies
| Panel | Cell Line | IGP (%) of Compound | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | 5j | ||
| Leukemia | CCRF-CEM | 31.62 | 11.56 | - | - | 3.41 | - | 25.24 | 13.73 | 6.77 | 10.91 |
| HL-60(TB) | 31.12 | 36.36 | 4.20 | * | * | * | * | 69.38 | * | * | |
| MOLT-4 | 16.23 | 17.07 | 4.05 | 12.96 | 3.64 | 10.64 | 23.59 | 9.06 | 0.44 | 24.25 | |
| RPMI-8226 | 24.20 | 2.85 | * | - | * | 0.99 | 18.21 | 81.00 | 1.18 | * | |
| SR | - | - | - | 21.80 | * | 53.71 | 26.54 | 1.76 | 6.36 | 22.08 | |
| NSCLC | HOP-62 | 15.32 | 13.98 | * | * | * | 6.75 | * | 34.63 | * | * |
| NCI-H226 | 13.09 | 26.50 | 6.62 | * | * | 17.09 | 3.34 | 14.78 | 1.10 | * | |
| NCI-H522 | - | - | 15.60 | * | 5.67 | 22.52 | 5.34 | 27.30 | * | * | |
| Colon cancer | HCC-2998 | - | - | * | * | * | 5.10 | * | 60.93 | * | * |
| HCT-116 | 9.47 | 2.28 | * | 0.68 | * | 3.30 | 7.37 | 43.60 | * | 8.42 | |
| SW-620 | * | 9.09 | * | * | * | * | * | 50.98 | * | * | |
| CNS cancer | SNB-19 | 6.57 | 9.45 | * | 4.26 | * | 17.47 | 9.70 | 3.40 | 2.53 | * |
| SNB-75 | 7.09 | - | - | 11.44 | 4.47 | - | 32.19 | 76.93 | 12.31 | 8.52 | |
| U251 | 12.89 | 16.48 | * | * | * | 17.55 | 5.97 | 21.68 | * | * | |
| Melanoma | LOX IMVI | 3.56 | 10.33 | * | 2.14 | * | 1.30 | 8.79 | 53.68 | 3.02 | * |
| UACC-257 | 11.40 | 1.35 | * | * | * | * | * | 33.69 | * | * | |
| UACC-62 | 16.61 | * | * | 4.08 | * | 5.83 | 0.29 | 23.80 | 2.39 | * | |
| Ovarian cancer | OVCAR-3 | * | 7.74 | * | * | * | * | * | 10.27 a | * | * |
| OVCAR-8 | 15.93 | 15.64 | * | 2.07 | * | 0.66 | 8.81 | 24.08 | 1.69 | * | |
| Renal cancer | SN12C | 2.62 | 14.33 | 1.69 | 4.46 | * | 17.11 | 11.87 | 15.54 | 1.69 | * |
| TK-10 | * | * | 5.41 | * | * | * | * | 52.33 | * | * | |
| UO-31 | 4.78 | 36.34 | 16.35 | 23.53 | 8.75 | 34.00 | 29.54 | 6.57 | 24.73 | 9.74 | |
| Prostate cancer | PC-3 | 19.12 | 11.45 | 1.37 | - | 6.74 | * | 1.87 | 51.71 | * | 0.36 |
| DU-145 | * | * | * | * | * | * | * | 98.79 | * | * | |
| Breast cancer | MCF-7 | 35.97 | 6.77 | 8.25 | 4.44 | * | 10.50 | 15.78 | 23.52 | 5.32 | 10.35 |
| MDA-MB-231/ATCC | 25.84 | 15.13 | 1.75 | * | * | * | * | 42.05 | * | * | |
| T-47D | 49.10 | 12.27 | * | * | * | * | 35.19 | 37.64 | * | * | |
| MDA-MB-468 | 53.37 | 18.73 | 5.58 | * | * | 9.62 | 4.93 | 47.18 a | * | * | |
| Cell Line | Equation | N | R | Rcv | s | RMSECV | p | F |
|---|---|---|---|---|---|---|---|---|
| MOLT-4 | IGP = 0.815(RDF055p) − 0.55(Mor32m) − 41.34 | 10 | 0.967 | 0.927 | 2.34 | 0.859 | 0.00006 | 51.34 |
| UO-31 | IGP = 0.770(Mor16e) − 0.497(RDF145m) + 67.048 | 10 | 0.943 | 0.861 | 11.44 | 1.371126 | 0.0006 | 28.33 |
| Compd. | MOLT-4 | UO-31 | ||
|---|---|---|---|---|
| RDF055p | Mor32m | Mor16e | RDF145m | |
| 5a | 5.903 | −0.701 | 1.632 | 3.349 |
| 5b | 6.251 | −0.775 | 1.243 | 5.546 |
| 5c | 4.882 | −0.627 | 1.529 | 3.010 |
| 5d | 5.670 | −0.616 | 1.744 | 5.816 |
| 5e | 6.278 | −0.202 | 1.765 | 3.392 |
| 5f | 6.038 | −0.486 | 1.252 | 3.104 |
| 5g | 8.458 | −0.549 | 1.260 | 3.370 |
| 5h | 5.958 | −0.448 | 1.662 | 1.985 |
| 5i | 5.521 | −0.400 | 1.289 | 3.139 |
| 5j | 7.891 | −0.551 | 1.565 | 1.986 |
| Compd. | IGP [%] | |||
|---|---|---|---|---|
| MOLT-4 | UO-31 | |||
| Observed | Predicted | Observed | Predicted | |
| 5a | 16.23 | 13.82 | 4.78 | 14.44 |
| 5b | 17.07 | 19.33 | 36.34 | 43.11 |
| 5c | 4.05 | 6.96 | 16.35 | 15.78 |
| 5d | 12.96 | 10.15 | 23.53 | 11.71 |
| 5e | 3.64 | 2.13 | 8.75 | 7.11 |
| 5f | 10.64 | 9.13 | 34 | 25.97 |
| 5g | 23.59 | 28.91 | 29.54 | 28.72 |
| 5h | 9.06 | 7.55 | 6.57 | 5.05 |
| 5i | 0.44 | 4.77 | 24.73 | 27.15 |
| 5j | 24.25 | 21.63 | 9.74 | 9.61 |
3. Discussion
3.1. Anticancer Activity
3.2. QSAR Studies
4. Experimental Section
4.1. General Information
4.2. Synthesis
General Procedure for the Preparation of 3-(R2-Amino)-7-(azol-2 or 5-yl)-6-chloro-1,1-dioxo-1,4,2-benzodithiazines 5a–j
4.3. In Vitro Anticancer Screening
4.4. Methodology of Molecular Modeling and QSAR Models Development
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brzozowski, Z.; Sławiński, J.; Angielski, S.; Szczepańska-Konkel, M. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. III Synthesis and diuretic properties of 3-(R,R1-phenyl)amino-6-chloro-7-methyl-1,1-dioxo-1,4,2-benzodithiazines. Acta Pol. Pharm. 1985, 42, 313–318. [Google Scholar] [PubMed]
- Brzozowski, Z.; Sławiński, J. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. IV Synthesis of some novel N-substituted 3-amino-6-chloro-7-methyl-1,1-dioxo-1,4,2-benzodithiazines. Acta. Pol. Pharm. 1985, 42, 319–325. [Google Scholar]
- Brzozowski, Z.; Sławiński, J.; Gajewski, F.; Angielski, S.; Hoppe, A. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. V Synthesis and diuretic properties of some novel 3-phenylamino-7-carboxy-1,1-dioxo-1,4,2-benzodithiazines. Acta Pol. Pharm. 1985, 42, 413–420. [Google Scholar] [PubMed]
- Brzozowski, Z.; Sławiński, J. Preparation Method for Novel 1,1-dioxo-3-Mercapto-1,4,2-benzodithiazines (Sposób Otrzymywania Nowych 1,1-Diokso-3-merkapto-1,4,2-benzoditiazyn). P.L. Patent 134567, 20 May 1986. [Google Scholar]
- Brzozowski, Z.; Sławiński, J.; Gajewski, F.; Angielski, S.; Hoppe, A.; Janiec, W.; Piekarska, T. Preparation Method for Novel 3-Amino-1,1-dioxo-1,4,2-benzodithiazines (Sposób Otrzymywania Nowych 3-Amino-1,1-diokso-1,4,2-benzoditiazyn). P.L. Patent 140677, 30 April 1988. [Google Scholar]
- Brzozowski, Z.; Sławiński, J.; Gajewski, F.; Pomarnacka, E.; Janiec, W.; Piekarska, T. Preparation Method for Novel 3-Amino-1,1-dioxo-1,4,2-benzodithiazine Derivatives (Sposób Otrzymywania Pochodnych 3-Amino-1,1-diokso-1,4,2-benzoditiazyny). P.L. Patent 141834, 30 November 1988. [Google Scholar]
- Brzozowski, Z.; Sławiński, J.; Janiec, W.; Cegieła, U.; Śliwiński, L.; Sedlak, I. 1,1-Dioxo-1,4,2-benzoithiazine derivatives. XII Synthesis and pharmacological properties of some 6-chloro-3-carboxyalkylamino-7-methyl-1,1-dioxo-1,4,2-benzodithiazines. Acta Pol. Pharm. Drug Res. 1992, 49, 75–79. [Google Scholar]
- Brzozowski, Z.; Sączewski, F.; Neamati, N. Synthesis, antitumor and anti-HIV activities of benzodithiazine-dioxides. Bioorg. Med. Chem. 2006, 14, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Sanchez, T.; Kuo, C.L.; Gdaniec, M.; Neamati, N. Synthesis, antiviral, and anti-HIV-1 integrase activities of 3-aroyl-1,1-dioxo-1,4,2-benzodithiazines. Bioorg. Med. Chem. 2004, 12, 3663–3672. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Sławiński, J.; Sanchez, T.; Neamati, N. Synthesis and anti-HIV-1 integrase activities of 3-aroyl-2,3-dihydro-1,1-dioxo-1,4,2-benzodithiazines. Eur. J. Med. Chem. 2009, 44, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Gdaniec, M. Synthesis, structural characterization and in vitro antitumor activity of novel 6-chloro-1,1-dioxo-1,4,2-benzodithiazie derivatives. Bioorg. Med. Chem. 2003, 11, 3673–3681. [Google Scholar] [CrossRef]
- Brzozowski, Z.; Sączewski, F.; Gdaniec, M. Synthesis, structural characterization and in vitro antitumor activity of 4-dimethylaminopyridinium (6-chloro-1,1-dioxo-1,4,2-benzodithiazin-3-yl)methanides. Eur. J. Med. Chem. 2003, 38, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Sławiński, J.; Bednarski, P.J.; Grünert, R.; Gdaniec, M. Synthesis, structural characterization, and in vitro antitumor activity of novel N-(6-chloro-1,1-dioxo-1,4,2-benzodithiazin-3-yl)arylsulfonamides. Bioorg. Med. Chem. 2007, 15, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Sławiński, J.; Żołnowska, B.; Brzozowski, Z.; Kawiak, A.; Belka, M.; Bączek, T. Synthesis and QSAR study of novel 6-chloro-3-(2-arylmethylene-1-methylhydrazino)-1,4,2-benzodithiazine 1,1-dioxide derivatives with anticancer activity. Molecules 2015, 20, 5754–5770. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F. A new type of mixed anhydride and its applications to the synthesis of 7-substituted 8-chloro-5,5-dioxoimidazo[1,2-b][1,4,2]benzodithiazines with in vitro antitumor activity. J. Med. Chem. 2002, 45, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Pomarnacka, E.; Gdaniec, M. Synthesis and anticancer activity of 2-amino-8-chloro-5,5-dioxo[1,2,4]triazolo[2,3-b][1,4,2]benzodithiazine derivatives. Bioorg. Med. Chem. 2003, 11, 1259–1267. [Google Scholar] [CrossRef]
- Pomarnacka, E.; Kornicka, A.; Kuchnio, A.; Heinrichs, M.; Grünert, R.; Gdaniec, M.; Bednarski, P.J. Synthesis, cytotoxicity testing, and structure-activity relationship of novel 6-chloro-7-(4-phenylimino-4H-3,1-benzoxazin-2-yl)-3-(substituted)-1,4,2-benzodithiazine 1,1-dioxides. Arch. Pharm. Chem. Life Sci. 2011, 344, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sławiński, J. Pochodne 1,1-diokso-1,4,2-benzoditiazyny. 1. Syntezy niektórych pochodnych 7-karboksy-3-merkapto-1,1-diokso-1,4,2-benzoditiazyny. Acta Pol. Pharm. 1984, 41, 5–13. [Google Scholar] [PubMed]
- Brzozowski, Z.; Sławiński, J.; Borowik, W.; Gajewski, F. Pochodne 2-merkaptobenzensulfonamidu. VII. Syntezy niektórych nowych 1,3-dialkilo-2-[2-merkapto-lub metylotio)-4-R1-5-(karboksy, metoksykarbonylo-lub cyjano) benzenosulfonylo]guanidyn. Acta Pol. Pharm. Drug Res. 1992, 49, 93–96. [Google Scholar]
- Brzozowski, Z.; Gajewski, F.; Sławiński, J.; Pomarnacka, E. Pochodne 1,1-diokso-1,4,2-benzoditiazyny. XIII. Syntezy chlorków i amidów kwasów 6-R1-3-metylotio-1,1-diokso-1,4,2-benzoditiazyno-7-karboksylowych. Acta Pol. Pharm. Drug Res. 1993, 50, 199–203. [Google Scholar]
- Sławiński, J.; Brożewicz, K.; Fruziński, A.; Główka, M.L. Synthesis and antitumor activity of novel N'(2-benzylthiobenzenesulfonyl)-1H-pyrazole-1-amidine derivatives. Heterocycles 2011, 83, 1093–1109. [Google Scholar] [CrossRef]
- Brożewicz, K.; Sławiński, J. 1-(2-Mercaptobenzenesulfonyl)-3-hydroxyguanidines—Novel potent antiproliferatives, synthesis and in vitro biological activity. Eur. J. Med. Chem. 2012, 55, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiero, D.A.; Monks, P.A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer Drug Discovery and Development Program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar] [PubMed]
- Boyd, M.R.; Paull, K.D. Some Practical Considerations and Applications of the National Cancer Institute in Vitro Anticancer Drug Discovery Screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 Human Tumour Cell line Anticancer Drug Screen. Nat. Rev. 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Sample Availability: Samples of the compounds 5a–j are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorzelska, A.; Sławiński, J.; Brożewicz, K.; Ulenberg, S.; Bączek, T. Novel 3-Amino-6-chloro-7-(azol-2 or 5-yl)-1,1-dioxo-1,4,2-benzodithiazine Derivatives with Anticancer Activity: Synthesis and QSAR Study. Molecules 2015, 20, 21960-21970. https://doi.org/10.3390/molecules201219821
Pogorzelska A, Sławiński J, Brożewicz K, Ulenberg S, Bączek T. Novel 3-Amino-6-chloro-7-(azol-2 or 5-yl)-1,1-dioxo-1,4,2-benzodithiazine Derivatives with Anticancer Activity: Synthesis and QSAR Study. Molecules. 2015; 20(12):21960-21970. https://doi.org/10.3390/molecules201219821
Chicago/Turabian StylePogorzelska, Aneta, Jarosław Sławiński, Kamil Brożewicz, Szymon Ulenberg, and Tomasz Bączek. 2015. "Novel 3-Amino-6-chloro-7-(azol-2 or 5-yl)-1,1-dioxo-1,4,2-benzodithiazine Derivatives with Anticancer Activity: Synthesis and QSAR Study" Molecules 20, no. 12: 21960-21970. https://doi.org/10.3390/molecules201219821