Selective C-Arylation of 2,5-Dibromo-3-hexylthiophene via Suzuki Cross Coupling Reaction and Their Pharmacological Aspects
Abstract
:1. Introduction
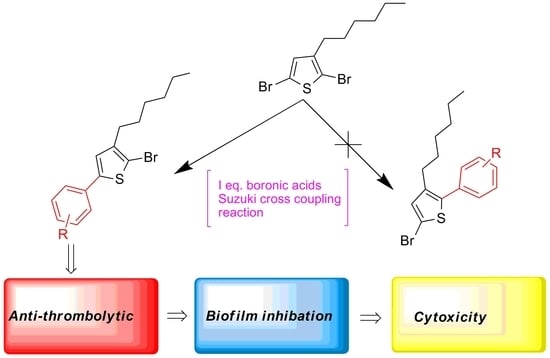
2. Results and Discussion
2.1. Chemistry

| Entry | Aryl Boronic Acids | Product | Solvent/H2O (4:1) | Yield% |
|---|---|---|---|---|
| 1 | 4-MeC6H4B(OH)2 |  | Dioxane | 76 |
| Toluene | 57 | |||
| 2 | 3,5-Me2C6H3B(OH)2 |  | Dioxane | 70 |
| 3 | 4-MeOC6H4B(OH)2 |  | Dioxane | 68 |
| 4 | 4-ClC6H4B(OH)2 |  | Dioxane | 77 |
| 5 | 4-IC6H4B(OH)2 |  | Dioxane | 69 |
| 6 | 3,5-F2C6H3B(OH)2 |  | Dioxane | 65 |
| 7 | 3-Cl,4-FC6H3B(OH)2 |  | Dioxane | 68 |
| 8 | 4-MeSC6H4B(OH)2 |  | Dioxane | 62 |
| 9 | 3-AcC6H4B(OH)2 |  | Dioxane | 72 |
2.2. Pharmacological Aspects
2.2.1. Haemolytic Activity
| Entry | % Lysis of RBC ± SD |
|---|---|
| 2a | 7.52 ± 0.042 |
| 2b | 4.54 ± 0.054 |
| 2c | 19.50 ± 0.079 |
| 2d | 9.13 ± 0.113 |
| 2e | 10.22 ± 0.084 |
| 2f | 6.55 ± 0.071 |
| 2g | 19.54 ± 0.095 |
| 2h | 5.97 ± 0.102 |
| 2i | 5.51 ± 0.089 |
| Positive Control | 100% |

2.2.2. Anti-Thrombolytic Activity
| Entry | % Lysis ± SD |
|---|---|
| 2a | 3.43 ± 0.022 |
| 2b | 7.58 ± 0.031 |
| 2c | 5.43 ± 0.015 |
| 2d | 26.75 ± 0.071 |
| 2e | 34.27 ± 0.019 |
| 2f | 14.54 ± 0.053 |
| 2g | 16.52 ± 0.035 |
| 2h | 3.6 ± 0.027 |
| 2i | 24.59 ± 0.046 |
| Positive control | 100 |
2.2.3. Biofilm Inhibition Assay
| Entry | % Inhibition ± SD |
|---|---|
| 2a | 66.75± 0.154 |
| 2b | 65.69± 0.134 |
| 2c | 52.12± 0.165 |
| 2d | 73.13± 0.154 |
| 2e | 67.02± 0.167 |
| 2f | 51.99± 0.188 |
| 2g | 75.66± 0.176 |
| 2h | 71.52± 0.163 |
| 2i | 68.48± 0.139 |
| Positive control | 97.43 |
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of 5-Aryl-2-Bromo-3-Hexylthiophene (2a–i)
3.3. Characterization Data
3.4. Haemolytic Activity
3.5. Biofilm Inhibition Assay
3.6. Anti-Thrombolytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyaura, N.; Yanagi, T.; Suzuki, A. The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases. Synth. Commun. 1981, 11, 513–519. [Google Scholar] [CrossRef]
- Paluru, D.K.; Dey, S.; Chaudhari, K.R.; Khedkar, M.V.; Bhanage, B.M.; Jain, V.K. Palladium(II) chalcogenolate complexes as catalysts for C-C cross-coupling and carbonylative Suzuki coupling reactions. Tetrahedron Lett. 2014, 55, 2953–2956. [Google Scholar] [CrossRef]
- Bellina, F.; Carpita, A.; Rossi, R. Palladium catalysts for the Suzuki cross-coupling reaction: An overview of recent advances. Synthesis 2004, 2004, 2419–2440. [Google Scholar] [CrossRef]
- Puterova, Z.; Krutosikova, A.; Vegh, D. Gewald reaction: Synthesis, properties and applications of substituted 2-aminothiophenes. ARKIVOC 2010, 1, 209–246. [Google Scholar] [CrossRef]
- Kheder, N.A.; Mabkhot, Y.N. Synthesis and antimicrobial studies of some novel bis-[1,3,4] thiadiazole and bis-thiazole pendant to thieno [2, 3-b] thiophene moiety. Int. J. Mol. Sci. 2012, 13, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, X.; Jenekhe, S.A. Thiophene-linked polyphenylquinoxaline: A new electron transport conjugated polymer for electroluminescent devices. Macromolecules 1999, 32, 3824–3826. [Google Scholar] [CrossRef]
- Steybe, F.; Effenberger, F.; Beckmann, S.; Kramer, P.; Glania, C.; Wortmann, R. Enhanced nonlinear optical properties and thermal stability of donor-acceptor substituted oligothiophenes. Chem. Phys. 1997, 219, 317–331. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, L.; Vasilevsky, S.F.; Verkruijsse, H.D. Application of Transition Metal Catalysts in Organic Synthesis; Springer-Verlag: Berlin/Heidelberg, Germary, 1999. [Google Scholar]
- Amatore, C.; Bahsoun, A.A.; Jutand, A.; Meyer, G.; Ntepe, N.; Ricard, L. Mechanism of the Stille reaction catalyzed by palladium ligated to arsine ligand: PhPdI (AsPh3)(DMF) is the species reacting with vinylstannane in DMF. J. Am. Chem. Soc. 2003, 125, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Rasool, N.; Dang, T.T.; Reinke, H.; Langer, P. Synthesis of tetraarylthiophenes by regioselective Suzuki cross-coupling reactions of tetrabromothiophene. Tetrahedron Lett. 2007, 48, 845–847. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Inada, K.; Miyaura, N. The Cross-Coupling Reaction of Arylboronic Acids with Chloropyridines and Electron-Deficient Chloroarenes Catalyzed by a Polymer-Bound Palladium Complex. Tetrahedron 2000, 56, 8661–8664. [Google Scholar] [CrossRef]
- Mologni, L.; Rostagno, R.; Brussolo, S.; Knowles, P.P.; Kjaer, S.; Murray-Rust, J.; Rosso, E.; Zambon, A.; Scapozza, L.; McDonald, N.Q. Synthesis, structure–activity relationship and crystallographic studies of 3-substituted indolin-2-one RET inhibitors. Bioorg. Med. Chem. 2010, 18, 1482–1496. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, Z.; Zhang, C.; Xin, T.; Wang, Y.; Song, H.; Jiang, Y.; Chen, Y.; Xu, Y.; Tan, C. Synthesis and Cytotoxic Activity of Some Novel N-Pyridinyl-2-(6-phenylimidazo [2, 1-b] thiazol-3-yl) acetamide Derivatives. Molecules 2012, 17, 4703–4716. [Google Scholar] [CrossRef] [PubMed]
- Allroggen, H.; Abbott, R.J. Cerebral venous sinus thrombosis. Postgrad. Med. J. 2000, 76, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Einhaupl, K.; Bousser, M.G.; de Bruijn, S.; Ferro, J.; Martinelli, I.; Masuhr, F.; Stam, J. EFNS guideline on the treatment of cerebral venous and sinus thrombosis. Eur. J. Neurol. 2006, 13, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Baruah, D.B.; Dash, R.N.; Chaudhari, M.; Kadam, S. Plasminogen activators: A comparison. Vasc. Pharmacol. 2006, 44, 1–9. [Google Scholar] [CrossRef]
- Haines, S.T.; Bussey, H.I. Thrombosis and the pharmacology of antithrombotic agents. Ann. Pharmacother. 1995, 29, 892–905. [Google Scholar] [PubMed]
- Sambanthamoorthy, K.; Gokhale, A.A.; Lao, W.; Parashar, V.; Neiditch, M.B.; Semmelhack, M.F.; Lee, I.; Waters, C.M. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 2011, 55, 4369–4378. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Ali, E.A.; Mokhtar, S.M.; Eweis, M. Synthesis, characterization polymerization and antibacterial properties of novel thiophene substituted acrylamide. React. Funct. Polym. 2011, 71, 1187–1194. [Google Scholar] [CrossRef]
- Ali, S.; Rasool, N.; Ullah, A.; Nasim, F.H.; Yaqoob, A.; Zubair, M.; Rashid, U.; Riaz, M. Design and Synthesis of Arylthiophene-2-Carbaldehydes via Suzuki-Miyaura Reactions and Their Biological Evaluation. Molecules 2013, 18, 14711–14725. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.; Catranis, C.; Maynard, C. Design of self-processing antimicrobial peptides for plant protection. Lett. Appl. Microbiol. 2000, 31, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Shahid, M.; Jamil, A. Sajjad-Ur-Rehman, Phytochemical Spectrum of Essential Oil of Paganum harmala by GC-MS and Antimicrobial Activity Using Sequential Solvents Fractions and Essential Oil. Asian J. Chem. 2014, 26, 574–578. [Google Scholar]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, H.M.; Rasool, N.; Ahmad, G.; Chotana, G.A.; Musharraf, S.G.; Zubair, M.; Rana, U.A.; Zia-Ul-Haq, M.; Jaafar, H.Z. Selective C-Arylation of 2,5-Dibromo-3-hexylthiophene via Suzuki Cross Coupling Reaction and Their Pharmacological Aspects. Molecules 2015, 20, 5202-5214. https://doi.org/10.3390/molecules20035202
Ikram HM, Rasool N, Ahmad G, Chotana GA, Musharraf SG, Zubair M, Rana UA, Zia-Ul-Haq M, Jaafar HZ. Selective C-Arylation of 2,5-Dibromo-3-hexylthiophene via Suzuki Cross Coupling Reaction and Their Pharmacological Aspects. Molecules. 2015; 20(3):5202-5214. https://doi.org/10.3390/molecules20035202
Chicago/Turabian StyleIkram, Hafiz Mansoor, Nasir Rasool, Gulraiz Ahmad, Ghayoor Abbas Chotana, Syed Ghulam Musharraf, Muhammad Zubair, Usman Ali Rana, Muhammad Zia-Ul-Haq, and Hawa Ze Jaafar. 2015. "Selective C-Arylation of 2,5-Dibromo-3-hexylthiophene via Suzuki Cross Coupling Reaction and Their Pharmacological Aspects" Molecules 20, no. 3: 5202-5214. https://doi.org/10.3390/molecules20035202