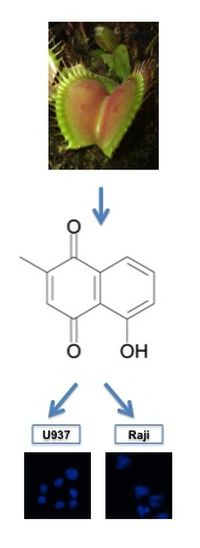
Plumbagin Modulates Leukemia Cell Redox Status
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plumbagin Reduces Leukemia Cell Viability
| Cell Lines | IC50 (µM) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| HL-60 | 1.38 ± 0.37 | 0.92 ± 0.16 | 0.90 ± 0.13 |
| Jurkat | 2.20 ± 1.07 | 0.98 ± 0.15 | 0.86 ± 0.16 |
| K562 | 1.07 ± 0.33 | 0.90 ± 0.32 | 0.89 ± 0.30 |
| Raji | 5.06 ± 0.22 | 3.49 ± 0.12 | 2.66 ± 0.03 |
| U937 | 0.82 ± 0.04 | 0.68 ± 0.01 | 0.66 ± 0.02 |
| PBMC | N.C. | N.C. | |
2.2. Plumbagin Induces Apoptotic Cell Death

2.3. Plumbagin Induces Different Levels of Intracellular ROS in U937 vs. Raji Cells


2.4. Differential ROS Generation Is not a Consequence of a Different Uptake/Efflux of Plumbagin

2.5. Thiol-Containing Antioxidants Prevent Plumbagin-Induced Apoptosis

2.6. Plumbagin Decreases the Intracellular GSH Level

| Treatment | Cell Lines | Control | H2O2 (50 µM) | Plumbagin (1 µM) | Plumbagin (5 µM) |
|---|---|---|---|---|---|
| Control (DMSO) | U937 | 100.00 | 88.38 ± 17.87 | 67.34 ± 9.21 (*) | 66.20 ± 13.26 (*) |
| Raji | 100.00 | 94.10 ± 13.88 | 88.49 ± 16.93 | 80.38 ± 5.59 (*) | |
| NAC (10 mM) | U937 | 100.00 | 89.99 ± 6.42 | 92.59 ± 17.54 | 85.71 ± 3.86 (**) |
| Raji | 100.00 | 111.36 ± 11.02 | 110.76 ± 8.74 | 90.85 ± 14.80 |
3. Experimental
3.1. Reagents
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Fluorescence Microscopy
3.5. Apoptosis Assays
3.6. Western-Blot
3.7. Evaluation of ROS Production
3.8. Plumbagin Intracellular Uptake and Efflux
3.9. Analysis of GSH Content
3.10. Statistical Analysis
4. Conclusions
| Cell Line | U937 | Raji | ||||||
|---|---|---|---|---|---|---|---|---|
| Plumbagin model | More sensitive (IC50 24 h = 1 μM) | Less sensitive (IC50 24 h = 5 μM) | ||||||
| Cell death | Apoptosis | |||||||
| Plumbagin uptake | Similar incorporation (up to 120 min) | |||||||
| Plumbagin efflux | No efflux, fluorescence remains constant | |||||||
| ROS production | Elevated | Moderate | ||||||
| GSH modulation | GSH modulation depending on their respective IC50 | |||||||
| Antioxidant classification | Thiol group | Non-thiol group | Thiol group | Non-thiol group | ||||
| Antioxidant | DTT | NAC | Tiron | Trolox | DTT | NAC | Tiron | Trolox |
| ROS production | ➘ | ➘➘ | ➙ | ➘ | ➙ | ➙ | ➙ | ➙ |
| Apoptosis | ➘ | ➘ | ➙ | ➙ | ➘ | ➘ | ➙ | ➙ |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Florean, C.; Schnekenburger, M.; Grandjenette, C.; Dicato, M.; Diederich, M. Epigenomics of leukemia: From mechanisms to therapeutic applications. Epigenomics 2011, 3, 581–609. [Google Scholar] [CrossRef]
- Karius, T.; Schnekenburger, M.; Dicato, M.; Diederich, M. Micrornas in cancer management and their modulation by dietary agents. Biochem. Pharmacol. 2012, 83, 1591–1601. [Google Scholar] [CrossRef]
- Schnekenburger, M.; Diederich, M. Epigenetics offer new horizons for colorectal cancer prevention. Curr. Colorectal Cancer Rep. 2012, 8, 66–81. [Google Scholar] [CrossRef]
- Gaascht, F.; Teiten, M.H.; Schumacher, M.; Dicato, M.; Diederich, M. Approche végétale dans le traitement des leucémies. Corresp. Onco-Hématol. 2010, V, 102–108. [Google Scholar]
- Teiten, M.H.; Gaascht, F.; Dicato, M.; Diederich, M. Anticancer bioactivity of compounds from medicinal plants used in european medieval traditions. Biochem. Pharmacol. 2013, 86, 1239–1247. [Google Scholar] [CrossRef]
- Sawadogo, W.R.; Schumacher, M.; Teiten, M.H.; Dicato, M.; Diederich, M. Traditional west african pharmacopeia, plants and derived compounds for cancer therapy. Biochem. Pharmacol. 2012, 84, 1225–1240. [Google Scholar] [CrossRef]
- Sawadogo, W.R.; Schumacher, M.; Teiten, M.H.; Cerella, C.; Dicato, M.; Diederich, M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2011. Molecules 2013, 18, 3641–3673. [Google Scholar] [CrossRef]
- Teiten, M.H.; Gaascht, F.; Eifes, S.; Dicato, M.; Diederich, M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010, 5, 61–74. [Google Scholar] [CrossRef]
- Teiten, M.H.; Eifes, S.; Reuter, S.; Duvoix, A.; Dicato, M.; Diederich, M. Gene expression profiling related to anti-inflammatory properties of curcumin in K562 leukemia cells. Ann. NY Acad. Sci. 2009, 1171, 391–398. [Google Scholar] [CrossRef]
- Teiten, M.H.; Eifes, S.; Dicato, M.; Diederich, M. Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins 2010, 2, 128–162. [Google Scholar] [CrossRef]
- Teiten, M.H.; Dicato, M.; Diederich, M. Curcumin as a regulator of epigenetic events. Mol. Nutr. Food Res. 2013, 57, 1619–1629. [Google Scholar] [CrossRef]
- Kelkel, M.; Cerella, C.; Mack, F.; Schneider, T.; Jacob, C.; Schumacher, M.; Dicato, M.; Diederich, M. ROS-independent JNK activation and multisite phosphorylation of Bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis 2012, 33, 2162–2171. [Google Scholar] [CrossRef]
- Czepukojc, B.; Baltes, A.K.; Cerella, C.; Kelkel, M.; Viswanathan, U.M.; Salm, F.; Burkholz, T.; Schneider, C.; Dicato, M.; Montenarh, M.; et al. Synthetic polysulfane derivatives induce cell cycle arrest and apoptotic cell death in human hematopoietic cancer cells. Food Chem. Toxicol. 2014, 64, 249–257. [Google Scholar] [CrossRef]
- Cerella, C.; Dicato, M.; Jacob, C.; Diederich, M. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer Agents Med. Chem. 2011, 11, 267–271. [Google Scholar] [CrossRef]
- Busch, C.; Jacob, C.; Anwar, A.; Burkholz, T.; Aicha Ba, L.; Cerella, C.; Diederich, M.; Brandt, W.; Wessjohann, L.; Montenarh, M. Diallylpolysulfides induce growth arrest and apoptosis. Int. J. Oncol. 2010, 36, 743–749. [Google Scholar]
- Slingerland, M.; Cerella, C.; Guchelaar, H.J.; Diederich, M.; Gelderblom, H. Cardiac glycosides in cancer therapy: From preclinical investigations towards clinical trials. Invest. New Drugs 2013, 31, 1087–1094. [Google Scholar] [CrossRef]
- Juncker, T.; Cerella, C.; Teiten, M.H.; Morceau, F.; Schumacher, M.; Ghelfi, J.; Gaascht, F.; Schnekenburger, M.; Henry, E.; Dicato, M.; et al. NBS1450, a steroid cardiac glycoside inducing apoptotic cell death in human leukemia cells. Biochem. Pharmacol. 2011, 81, 13–23. [Google Scholar] [CrossRef]
- Cerella, C.; Dicato, M.; Diederich, M. Assembling the puzzle of anti-cancer mechanisms triggered by cardiac glycosides. Mitochondrion 2013, 13, 225–234. [Google Scholar] [CrossRef]
- Ebrahim, W.; Aly, A.H.; Wray, V.; Mandi, A.; Teiten, M.H.; Gaascht, F.; Orlikova, B.; Kassack, M.U.; Lin, W.; Diederich, M.; et al. Embellicines A and B: Absolute configuration and NF-κB transcriptional inhibitory activity. J. Med. Chem. 2013, 56, 2991–2999. [Google Scholar] [CrossRef]
- Teiten, M.H.; Mack, F.; Debbab, A.; Aly, A.H.; Dicato, M.; Proksch, P.; Diederich, M. Anticancer effect of altersolanol a, a metabolite produced by the endophytic fungus stemphylium globuliferum, mediated by its pro-apoptotic and anti-invasive potential via the inhibition of NF-κB activity. Bioorg. Med. Chem. 2013, 21, 3850–3858. [Google Scholar] [CrossRef]
- Aung, H.H.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Ahmed, A.A.; Pare, P.W.; Mabry, T.J. Phenolic constituents from the leaves of the carnivorous plant nepenthes gracilis. Fitoterapia 2002, 73, 445–447. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic action of juglone and plumbagin: A mechanistic study using hacat keratinocytes. Chem. Res. Toxicol. 2004, 17, 55–62. [Google Scholar] [CrossRef]
- Ahmad, A.; Banerjee, S.; Wang, Z.; Kong, D.; Sarkar, F.H. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-κB and Bcl-2. J. Cell Biochem. 2008, 105, 1461–1471. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Cho, C.Y.; Kuo, P.L.; Huang, Y.T.; Lin, C.C. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation at serine 15 in vitro and in vivo. J. Pharmacol. Exp. Ther. 2006, 318, 484–494. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Rajendran, P.; Li, F.; Ramachandran, L.; Hay, H.S.; Kannaiyan, R.; Swamy, S.N.; Vali, S.; Kapoor, S.; et al. Plumbagin inhibits invasion and migration of breast and gastric cancer cells by downregulating the expression of chemokine receptor CXCR4. Mol. Cancer 2011, 10, 107. [Google Scholar] [CrossRef]
- Powolny, A.A.; Singh, S.V. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm. Res. 2008, 25, 2171–2180. [Google Scholar] [CrossRef]
- Srinivas, P.; Gopinath, G.; Banerji, A.; Dinakar, A.; Srinivas, G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol. Carcinog. 2004, 40, 201–211. [Google Scholar] [CrossRef]
- Wang, C.C.; Chiang, Y.M.; Sung, S.C.; Hsu, Y.L.; Chang, J.K.; Kuo, P.L. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008, 259, 82–98. [Google Scholar]
- Gomathinayagam, R.; Sowmyalakshmi, S.; Mardhatillah, F.; Kumar, R.; Akbarsha, M.A.; Damodaran, C. Anticancer mechanism of plumbagin, a natural compound, on non-small cell lung cancer cells. Anticancer Res. 2008, 28, 785–792. [Google Scholar]
- Lee, J.H.; Yeon, J.H.; Kim, H.; Roh, W.; Chae, J.; Park, H.O.; Kim, D.M. The natural anticancer agent plumbagin induces potent cytotoxicity in MCF-7 human breast cancer cells by inhibiting a PI-5 kinase for ros generation. PLoS One 2012, 7, e45023. [Google Scholar]
- Nazeem, S.; Azmi, A.S.; Hanif, S.; Ahmad, A.; Mohammad, R.M.; Hadi, S.M.; Kumar, K.S. Plumbagin induces cell death through a copper-redox cycle mechanism in human cancer cells. Mutagenesis 2009, 24, 413–418. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Aggarwal, B.B. 5-hydroxy-2-methyl-1,4-naphthoquinone, a vitamin K3 analogue, suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase, SHP-1: Potential role in chemosensitization. Mol. Cancer Res. 2010, 8, 107–118. [Google Scholar] [CrossRef]
- Subramaniya, B.R.; Srinivasan, G.; Sadullah, S.S.; Davis, N.; Subhadara, L.B.; Halagowder, D.; Sivasitambaram, N.D. Apoptosis inducing effect of plumbagin on colonic cancer cells depends on expression of COX-2. PLoS One 2011, 6, e18695. [Google Scholar] [CrossRef]
- Sun, J.; McKallip, R.J. Plumbagin treatment leads to apoptosis in human K562 leukemia cells through increased ROS and elevated TRAIL receptor expression. Leuk. Res. 2011, 35, 1402–1408. [Google Scholar] [CrossRef]
- Lai, L.; Liu, J.; Zhai, D.; Lin, Q.; He, L.; Dong, Y.; Zhang, J.; Lu, B.; Chen, Y.; Yi, Z.; et al. Plumbagin inhibits tumour angiogenesis and tumour growth through the RAS signalling pathway following activation of the VEGF receptor-2. Br. J. Pharmacol. 2012, 165, 1084–1096. [Google Scholar] [CrossRef]
- Sinha, S.; Pal, K.; Elkhanany, A.; Dutta, S.; Cao, Y.; Mondal, G.; Iyer, S.; Somasundaram, V.; Couch, F.J.; Shridhar, V.; et al. Plumbagin inhibits tumorigenesis and angiogenesis of ovarian cancer cells in vivo. Int. J. Cancer 2013, 132, 1201–1212. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-κB activation and NF-κB-regulated gene products through modulation of p65 and ikappabalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J. Biol. Chem. 2006, 281, 17023–17033. [Google Scholar] [CrossRef]
- Xu, T.P.; Shen, H.; Liu, L.X.; Shu, Y.Q. Plumbagin from plumbago zeylanica l induces apoptosis in human non-small cell lung cancer cell lines through NF-κB inactivation. Asian Pac. J. Cancer Prev. 2013, 14, 2325–2331. [Google Scholar] [CrossRef]
- Yang, S.J.; Chang, S.C.; Wen, H.C.; Chen, C.Y.; Liao, J.F.; Chang, C.H. Plumbagin activates ERK1/2 and AKT via superoxide, SRC and PI3-kinase in 3T3-l1 cells. Eur. J. Pharmacol. 2010, 638, 21–28. [Google Scholar]
- Dhar, S.K.; Tangpong, J.; Chaiswing, L.; Oberley, T.D.; St Clair, D.K. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res. 2011, 71, 6684–6695. [Google Scholar] [CrossRef]
- Lincoln, D.T.; Ali Emadi, E.M.; Tonissen, K.F.; Clarke, F.M. The thioredoxin-thioredoxin reductase system: Over-expression in human cancer. Anticancer Res. 2003, 23, 2425–2433. [Google Scholar]
- Perry, R.R.; Mazetta, J.A.; Levin, M.; Barranco, S.C. Glutathione levels and variability in breast tumors and normal tissue. Cancer 1993, 72, 783–787. [Google Scholar] [CrossRef]
- Skrzycki, M.; Majewska, M.; Podsiad, M.; Czeczot, H. Expression and activity of superoxide dismutase isoenzymes in colorectal cancer. Acta Biochim. Pol. 2009, 56, 663–670. [Google Scholar]
- Zhou, J.; Du, Y. Acquisition of resistance of pancreatic cancer cells to 2-methoxyestradiol is associated with the upregulation of manganese superoxide dismutase. Mol. Cancer Res. 2012, 10, 768–777. [Google Scholar] [CrossRef]
- Checker, R.; Sharma, D.; Sandur, S.K.; Subrahmanyam, G.; Krishnan, S.; Poduval, T.B.; Sainis, K.B. Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J. Cell Biochem. 2010, 110, 1082–1093. [Google Scholar] [CrossRef]
- Castro, F.A.; Mariani, D.; Panek, A.D.; Eleutherio, E.C.; Pereira, M.D. Cytotoxicity mechanism of two naphthoquinones (menadione and plumbagin) in saccharomyces cerevisiae. PLoS One 2008, 3, e3999. [Google Scholar] [CrossRef]
- SivaKumar, V.; Prakash, R.; Murali, M.R.; Devaraj, H.; Niranjali Devaraj, S. In vivo micronucleus assay and gst activity in assessing genotoxicity of plumbagin in swiss albino mice. Drug Chem. Toxicol. 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Srinivasan, L.; Mathew, N.; Muthuswamy, K. In vitro antifilarial activity of glutathione S-transferase inhibitors. Parasitol. Res. 2009, 105, 1179–1182. [Google Scholar] [CrossRef]
- Seshadri, P.; Rajaram, A.; Rajaram, R. Plumbagin and juglone induce caspase-3-dependent apoptosis involving the mitochondria through ROS generation in human peripheral blood lymphocytes. Free Radic. Biol. Med. 2011, 51, 2090–2107. [Google Scholar] [CrossRef]
- Cerella, C.; Sobolewski, C.; Chateauvieux, S.; Henry, E.; Schnekenburger, M.; Ghelfi, J.; Dicato, M.; Diederich, M. Cox-2 inhibitors block chemotherapeutic agent-induced apoptosis prior to commitment in hematopoietic cancer cells. Biochem. Pharmacol. 2011, 82, 1277–1290. [Google Scholar] [CrossRef]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Seo, H.R.; Seo, W.D.; Pyun, B.J.; Lee, B.W.; Jin, Y.B.; Park, K.H.; Seo, E.K.; Lee, Y.J.; Lee, Y.S. Radiosensitization by celastrol is mediated by modification of antioxidant thiol molecules. Chem. Biol. Interact. 2011, 193, 34–42. [Google Scholar] [CrossRef]
- Monticone, M.; Taherian, R.; Stigliani, S.; Carra, E.; Monteghirfo, S.; Longo, L.; Daga, A.; Dono, M.; Zupo, S.; Giaretti, W.; et al. NAC, tiron and trolox impair survival of cell cultures containing glioblastoma tumorigenic initiating cells by inhibition of cell cycle progression. PLoS One 2014, 9, e90085. [Google Scholar]
- Yang, J.; Su, Y.; Richmond, A. Antioxidants tiron and N-acetyl-l-cysteine differentially mediate apoptosis in melanoma cells via a reactive oxygen species-independent NF-κB pathway. Free Radic. Biol. Med. 2007, 42, 1369–1380. [Google Scholar] [CrossRef]
- Arrigo, A.P. Gene expression and the thiol redox state. Free Radic. Biol. Med. 1999, 27, 936–944. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Chen, C.; Zeng, Z.; Yang, H.; Oh, J.; Chen, L.; Lu, S.C. Mechanism and significance of increased glutathione level in human hepatocellular carcinoma and liver regeneration. FASEB J. 2001, 15, 19–21. [Google Scholar]
- Warner, B.B.; Stuart, L.; Gebb, S.; Wispe, J.R. Redox regulation of manganese superoxide dismutase. Am. J. Physiol. 1996, 271, L150–L158. [Google Scholar]
- Franco, R.; Cidlowski, J.A. Glutathione efflux and cell death. Antioxid. Redox Signaling 2012, 17, 1694–1713. [Google Scholar] [CrossRef]
- Schumacher, M.; Cerella, C.; Eifes, S.; Chateauvieux, S.; Morceau, F.; Jaspars, M.; Dicato, M.; Diederich, M. Heteronemin, a spongean sesterterpene, inhibits tnf alpha-induced NF-kappa b activation through proteasome inhibition and induces apoptotic cell death. Biochem. Pharmacol. 2010, 79, 610–622. [Google Scholar] [CrossRef]
- D’Alessio, M.; Cerella, C.; Amici, C.; Pesce, C.; Coppola, S.; Fanelli, C.; de Nicola, M.; Cristofanon, S.; Clavarino, G.; Bergamaschi, A.; et al. Glutathione depletion up-regulates BCL-2 in BSO-resistant cells. FASEB J. 2004, 18, 1609–1611. [Google Scholar]
- Acharya, B.R.; Bhattacharyya, B.; Chakrabarti, G. The natural naphthoquinone plumbagin exhibits antiproliferative activity and disrupts the microtubule network through tubulin binding. Biochemistry 2008, 47, 7838–7845. [Google Scholar] [CrossRef]
- Ghibelli, L.; Diederich, M. Multistep and multitask bax activation. Mitochondrion 2010, 10, 604–613. [Google Scholar] [CrossRef]
- Nie, C.; Tian, C.; Zhao, L.; Petit, P.X.; Mehrpour, M.; Chen, Q. Cysteine 62 of bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J. Biol. Chem. 2008, 283, 15359–15369. [Google Scholar]
- Honda, T.; Coppola, S.; Ghibelli, L.; Cho, S.H.; Kagawa, S.; Spurgers, K.B.; Brisbay, S.M.; Roth, J.A.; Meyn, R.E.; Fang, B.; et al. GSH depletion enhances adenoviral bax-induced apoptosis in lung cancer cells. Cancer Gene Ther. 2004, 11, 249–255. [Google Scholar] [CrossRef]
- D’Alessio, M.; de Nicola, M.; Coppola, S.; Gualandi, G.; Pugliese, L.; Cerella, C.; Cristofanon, S.; Civitareale, P.; Ciriolo, M.R.; Bergamaschi, A.; et al. Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J. 2005, 19, 1504–1506. [Google Scholar]
- Turella, P.; Cerella, C.; Filomeni, G.; Bullo, A.; de Maria, F.; Ghibelli, L.; Ciriolo, M.R.; Cianfriglia, M.; Mattei, M.; Federici, G.; et al. Proapoptotic activity of new glutathione S-transferase inhibitors. Cancer Res. 2005, 65, 3751–3761. [Google Scholar] [CrossRef]
- Duvoix, A.; Schnekenburger, M.; Delhalle, S.; Blasius, R.; Borde-Chiche, P.; Morceau, F.; Dicato, M.; Diederich, M. Expression of glutathione S-transferase P1–1 in leukemic cells is regulated by inducible AP-1 binding. Cancer Lett. 2004, 216, 207–219. [Google Scholar]
- Karius, T.; Schnekenburger, M.; Ghelfi, J.; Walter, J.; Dicato, M.; Diederich, M. Reversible epigenetic fingerprint-mediated glutathione-s-transferase P1 gene silencing in human leukemia cell lines. Biochem. Pharmacol. 2011, 81, 1329–1342. [Google Scholar] [CrossRef]
- Sample Availability: Plumbagin is a commercially available compound as reported in the “Experimental” section.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaascht, F.; Teiten, M.-H.; Cerella, C.; Dicato, M.; Bagrel, D.; Diederich, M. Plumbagin Modulates Leukemia Cell Redox Status. Molecules 2014, 19, 10011-10032. https://doi.org/10.3390/molecules190710011
Gaascht F, Teiten M-H, Cerella C, Dicato M, Bagrel D, Diederich M. Plumbagin Modulates Leukemia Cell Redox Status. Molecules. 2014; 19(7):10011-10032. https://doi.org/10.3390/molecules190710011
Chicago/Turabian StyleGaascht, François, Marie-Hélène Teiten, Claudia Cerella, Mario Dicato, Denyse Bagrel, and Marc Diederich. 2014. "Plumbagin Modulates Leukemia Cell Redox Status" Molecules 19, no. 7: 10011-10032. https://doi.org/10.3390/molecules190710011