Long-Term Leaching Behavior and Geochemical Modeling of Cement Solidified Incineration Fly Ash Containing Waste Tires and Wood Biomass
Abstract
:1. Introduction
2. Research Method
2.1. Solidified Boiler Fly Ash
2.2. Test Road Pilot Site Samples
2.3. Chemical and Mineralogical Analysis
2.4. Batch Leaching Tests
2.5. Column Experiments
2.6. Reactive Transport Model in Saturated Conditions
3. Results and Discussion
3.1. Characterization of Samples
3.2. Compliance Batch Leaching Test: JLT-46
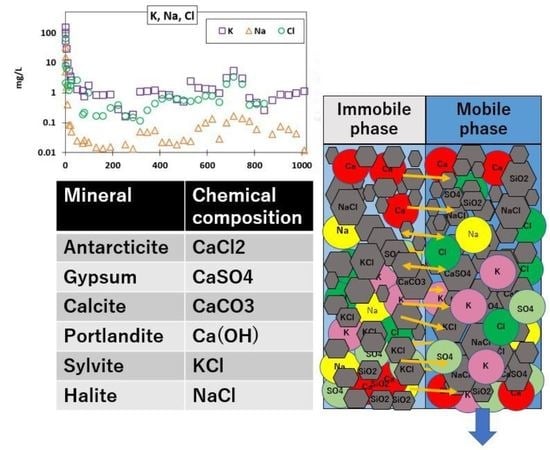
3.3. Column Leaching Test
3.4. pH-Stat Leaching Test
3.5. Column Leaching Modeling Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Papageorgiou, A.; Barton, J.R.; Karagiannidis, A. Assessment of the Greenhouse Effect Impact of Technologies Used for Energy Recovery from Municipal Waste: A Case for England. J. Environ. Manag. 2009, 90, 2999–3012. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Robert, D.; Mohajerani, A.; Tran, P.; Pramanik, B.K. Utilisation of Waste-to-Energy Fly Ash in Ceramic Tiles. Constr. Build. Mater. 2022, 347, 128475. [Google Scholar] [CrossRef]
- Birgisdóttir, H.; Bhander, G.; Hauschild, M.Z.; Christensen, T.H. Life Cycle Assessment of Disposal of Residues from Municipal Solid Waste Incineration: Recycling of Bottom Ash in Road Construction or Landfilling in Denmark Evaluated in the ROAD-RES Model. Waste Manag. 2007, 27, S75–S84. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Abis, M.; Kuchta, K.; Simon, F.-G.; Grönholm, R.; Hoppe, M.; Fiore, S. Material Flow, Economic and Environmental Assessment of Municipal Solid Waste Incineration Bottom Ash Recycling Potential in Europe. J. Clean. Prod. 2021, 317, 128511. [Google Scholar] [CrossRef]
- Huber, F.; Fellner, J. Integration of Life Cycle Assessment with Monetary Valuation for Resource Classification: The Case of Municipal Solid Waste Incineration Fly Ash. Resour. Conserv. Recycl. 2018, 139, 17–26. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Lynn, C.J.; Dhir, R.K. Environmental Impacts of the Use of Bottom Ashes from Municipal Solid Waste Incineration: A Review. Resour. Conserv. Recycl. 2019, 140, 23–35. [Google Scholar] [CrossRef]
- Meskhi, B.; Beskopylny, A.N.; Stel’makh, S.A.; Shcherban’, E.M.; Mailyan, L.R.; Shilov, A.A.; El’shaeva, D.; Shilova, K.; Karalar, M.; Aksoylu, C.; et al. Analytical Review of Geopolymer Concrete: Retrospective and Current Issues. Materials 2023, 16, 3792. [Google Scholar] [CrossRef]
- Çelik, A.İ.; Tunç, U.; Bahrami, A.; Karalar, M.; Othuman Mydin, M.A.; Alomayri, T.; Özkılıç, Y.O. Use of Waste Glass Powder toward More Sustainable Geopolymer Concrete. J. Mater. Res. Technol. 2023, 24, 8533–8546. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A Review on the Utilization of Fly Ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Almahayni, T.; Vanhoudt, N. Does Leaching of Naturally Occurring Radionuclides from Roadway Pavements Stabilised with Coal Fly Ash Have Negative Impacts on Groundwater Quality and Human Health? J. Hazard. Mater. 2018, 349, 128–134. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, Y.J.; Kim, H.J.; Lee, J.J. Performance Evaluation of the Use of Tire-Derived Fuel Fly Ash as Mineral Filler in Hot Mix Asphalt Concrete. J. Traffic Transp. Eng. 2020, 7, 249–258. [Google Scholar] [CrossRef]
- Hjelmar, O.; Holm, J.; Crillesen, K. Utilisation of MSWI Bottom Ash as Sub-Base in Road Construction: First Results from a Large-Scale Test Site. J. Hazard. Mater. 2007, 139, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Soleimanbeigi, A.; Likos, W.J.; Edil, T.B. Geotechnical and Leaching Properties of Municipal Solid Waste Incineration Fly Ash for Use as Embankment Fill Material. Transp. Res. Rec. 2016, 2579, 70–78. [Google Scholar] [CrossRef]
- Clavier, K.A.; Paris, J.M.; Ferraro, C.C.; Townsend, T.G. Opportunities and Challenges Associated with Using Municipal Waste Incineration Ash as a Raw Ingredient in Cement Production—A Review. Resour. Conserv. Recycl. 2020, 160, 104888. [Google Scholar] [CrossRef]
- Li, J. Municipal Solid Waste Incineration Ash-Incorporated Concrete: One Step towards Environmental Justice. Buildings 2021, 11, 495. [Google Scholar] [CrossRef]
- Zhang, Y.; Maierdan, Y.; Guo, T.; Chen, B.; Fang, S.; Zhao, L. Biochar as Carbon Sequestration Material Combines with Sewage Sludge Incineration Ash to Prepare Lightweight Concrete. Constr. Build. Mater. 2022, 343, 128116. [Google Scholar] [CrossRef]
- Ogawa, N.; Amano, T.; Nagai, Y.; Hagiwara, K.; Honda, T.; Koike, Y. Water Repellents for the Leaching Control of Heavy Metals in Municipal Solid Waste Incineration Fly Ash. Waste Manag. 2021, 124, 154–159. [Google Scholar] [CrossRef]
- Bie, R.; Chen, P.; Song, X.; Ji, X. Characteristics of Municipal Solid Waste Incineration Fly Ash with Cement Solidification Treatment. J. Energy Inst. 2016, 89, 704–712. [Google Scholar] [CrossRef]
- Czop, M.; Łaźniewska-Piekarczyk, B. Evaluation of the Leachability of Contaminations of Fly Ash and Bottom Ash from the Combustion of Solid Municipal Waste before and after Stabilization Process. Sustainability 2019, 11, 5384. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zeng, M.; Ji, W. Characteristics of the Cement-Solidified Municipal Solid Waste Incineration Fly Ash. Env. Sci Pollut. Res. 2018, 25, 36736–36744. [Google Scholar] [CrossRef]
- Shao, Y.; Hou, H.; Wang, G.; Wan, S.; Zhou, M. Characteristics of the Stabilized/Solidified Municipal Solid Wastes Incineration Fly Ash and the Leaching Behavior of Cr and Pb. Front. Environ. Sci. Eng. 2016, 10, 192–200. [Google Scholar] [CrossRef]
- Yakubu, Y.; Zhou, J.; Ping, D.; Shu, Z.; Chen, Y. Effects of PH Dynamics on Solidification/Stabilization of Municipal Solid Waste Incineration Fly Ash. J. Environ. Manag. 2018, 207, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Fellner, J.; Döberl, G.; Allgaier, G.; Brunner, P.H. Comparing Field Investigations with Laboratory Models to Predict Landfill Leachate Emissions. Waste Manag. 2009, 29, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Fellner, J.; Brunner, P.H. Modeling of Leachate Generation from MSW Landfills by a 2-Dimensional 2-Domain Approach. Waste Manag. 2010, 30, 2084–2095. [Google Scholar] [CrossRef]
- Hyks, J.; Astrup, T.; Christensen, T.H. Long-Term Leaching from MSWI Air-Pollution-Control Residues: Leaching Characterization and Modeling. J. Hazard. Mater. 2009, 162, 80–91. [Google Scholar] [CrossRef]
- Dabo, D.; Badreddine, R.; De Windt, L.; Drouadaine, I. Ten-Year Chemical Evolution of Leachate and Municipal Solid Waste Incineration Bottom Ash Used in a Test Road Site. J. Hazard. Mater. 2009, 172, 904–913. [Google Scholar] [CrossRef]
- Du, B.; Li, J.; Fang, W.; Liu, J. Comparison of Long-Term Stability under Natural Ageing between Cement Solidified and Chelator-Stabilised MSWI Fly Ash. Environ. Pollut. 2019, 250, 68–78. [Google Scholar] [CrossRef]
- Du, B.; Li, J.; Fang, W.; Liu, Y.; Yu, S.; Li, Y.; Liu, J. Characterization of Naturally Aged Cement-Solidified MSWI Fly Ash. Waste Manag. 2018, 80, 101–111. [Google Scholar] [CrossRef]
- Klich, I.; Batchelor, B.; Wilding, L.P.; Drees, L.R. Mineralogical Alterations That Affect the Durability and Metals Containment of Aged Solidified and Stabilized Wastes. Cem. Concr. Res. 1999, 29, 1433–1440. [Google Scholar] [CrossRef]
- Pandey, B.; Kinrade, S.D.; Catalan, L.J.J. Effects of Carbonation on the Leachability and Compressive Strength of Cement-Solidified and Geopolymer-Solidified Synthetic Metal Wastes. J. Environ. Manag. 2012, 101, 59–67. [Google Scholar] [CrossRef]
- Dahim, M.; Abuaddous, M.; Al-Mattarneh, H.; Rawashdeh, A.; Ismail, R. Enhancement of Road Pavement Material Using Conventional and Nano-Crude Oil Fly Ash. Appl. Nanosci. 2021, 11, 2517–2524. [Google Scholar] [CrossRef]
- James, A.K.; Thring, R.W.; Helle, S.; Ghuman, H.S. Ash Management Review—Applications of Biomass Bottom Ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, J.; Lu, J.; Cheeseman, C.; Poon, C.S. Recycling Incinerated Sewage Sludge Ash (ISSA) as a Cementitious Binder by Lime Activation. J. Clean. Prod. 2020, 244, 118856. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Wang, S.; Formela, K. Waste Tire Rubber Devulcanization Technologies: State-of-the-Art, Limitations and Future Perspectives. Waste Manag. 2022, 150, 174–184. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Chen, C.-C.; Lin, Y.-Q.; Hsiao, C.-F.; Tsai, C.-H.; Hsieh, M.-H. Status of Waste Tires’ Recycling for Material and Energy Resources in Taiwan. J. Mater. Cycles Waste Manag. 2017, 19, 1288–1294. [Google Scholar] [CrossRef]
- Feraldi, R.; Cashman, S.; Huff, M.; Raahauge, L. Comparative LCA of Treatment Options for US Scrap Tires: Material Recycling and Tire-Derived Fuel Combustion. Int. J. Life Cycle Assess. 2013, 18, 613–625. [Google Scholar] [CrossRef]
- Pan, D.; Jiang, W.; Guo, R.; Huang, Y.; Pan, W. Thermogravimetric and Kinetic Analysis of Co-Combustion of Waste Tires and Coal Blends. ACS Omega 2021, 6, 5479–5484. [Google Scholar] [CrossRef]
- Pipilikaki, P.; Katsioti, M.; Papageorgiou, D.; Fragoulis, D.; Chaniotakis, E. Use of Tire Derived Fuel in Clinker Burning. Cem. Concr. Compos. 2005, 27, 843–847. [Google Scholar] [CrossRef]
- Ćetković, J.; Lakić, S.; Žarković, M.; Vujadinović, R.; Knežević, M.; Živković, A.; Cvijović, J. Environmental Benefits of Air Emission Reduction in the Waste Tire Management Practice. Processes 2022, 10, 787. [Google Scholar] [CrossRef]
- Mentes, D.; Tóth, C.E.; Nagy, G.; Muránszky, G.; Póliska, C. Investigation of Gaseous and Solid Pollutants Emitted from Waste Tire Combustion at Different Temperatures. Waste Manag. 2022, 149, 302–312. [Google Scholar] [CrossRef]
- Banar, M.; Özkan, A.; Akyıldız, V.; Çokaygil, Z.; Onay, Ö. Evaluation of Solid Product Obtained from Tire-Derived Fuel (TDF) Pyrolysis as Carbon Black. J. Mater. Cycles Waste Manag. 2015, 17, 125–134. [Google Scholar] [CrossRef]
- Hower, J.C.; Robertson, J.D. Chemistry and Petrology of Fly Ash Derived from the Co-Combustion of Western United States Coal and Tire-Derived Fuel. Fuel Process. Technol. 2004, 85, 359–377. [Google Scholar] [CrossRef]
- Raudonytė-Svirbutavičienė, E.; Stakėnienė, R.; Jokšas, K.; Valiulis, D.; Byčenkienė, S.; Žarkov, A. Distribution of Polycyclic Aromatic Hydrocarbons and Heavy Metals in Soil Following a Large Tire Fire Incident: A Case Study. Chemosphere 2022, 286, 131556. [Google Scholar] [CrossRef] [PubMed]
- Çelik, A.; Ozk, Y.O. Geopolymer Concrete with High Strength, Workability and Setting Time Using Recycled Steel Wires and Basalt Powder. Steel Compos. Struct. 2023, 46, 689–707. [Google Scholar]
- Zeybek, Ö.; Özkılıç, Y.O.; Çelik, A.İ.; Deifalla, A.F.; Ahmad, M.; Sabri Sabri, M.M. Performance Evaluation of Fiber-Reinforced Concrete Produced with Steel Fibers Extracted from Waste Tire. Front. Mater. 2022, 9, 692. [Google Scholar] [CrossRef]
- Paul, S.; Rahaman, M.; Ghosh, S.K.; Katheria, A.; Das, T.K.; Patel, S.; Das, N.C. Recycling of Waste Tire by Pyrolysis to Recover Carbon Black: An Alternative Reinforcing Filler. J. Mater. Cycles Waste Manag. 2023, 25, 1470–1481. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kinoshita, T.; Akita, S. Thermal Treatment of Waste Tire Fly Ash with Polyvinyl Chloride: Selective Leaching of Zinc with Water. Ind. Eng. Chem. Res. 2006, 45, 1211–1216. [Google Scholar] [CrossRef]
- Li, S.; Tran, T.Q.; Li, Q.; Ji, B.; Brand, A.S.; Zhang, W. Zn Leaching Recovery and Mechanisms from End-of-Life Tire Rubber. Resour. Conserv. Recycl. 2023, 194, 107004. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Krzyżyńska, R.; Jouhara, H.; Spencer, N. Use of Pyrolytic Gas from Waste Tire as a Fuel: A Review. Energy 2017, 134, 1121–1131. [Google Scholar] [CrossRef]
- Santiago, J.R.; Sekito, T.; Dote, Y. Leaching Behavior and Mineral Speciation of Cement-Solidified Boiler Fly Ash from Industrial Waste Incineration Containing Waste Tires. J. Mater. Cycles Waste Manag. 2022, 25, 910–919. [Google Scholar] [CrossRef]
- Itabashi, T.; Li, J.; Hashimoto, Y.; Ueshima, M.; Sakanakura, H.; Yasutaka, T.; Imoto, Y.; Hosomi, M. Speciation and Fractionation of Soil Arsenic from Natural and Anthropogenic Sources: Chemical Extraction, Scanning Electron Microscopy, and Micro-XRF/XAFS Investigation. Environ. Sci. Technol. 2019, 53, 14186–14193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, D.; Šimůnek, J.; Mallants, D.; van Genuchten, M.T. Modelling Coupled Water Flow, Solute Transport and Geochemical Reactions Affecting Heavy Metal Migration in a Podzol Soil. Geoderma 2008, 145, 449–461. [Google Scholar] [CrossRef]
- Šimůnek, J.; Jacques, D.; Van Genuchten, M.T.; Mallants, D. Multicomponent Geochemical Transport Modeling Using Hydrus-1d and Hp11. JAWRA J. Am. Water Resour. Assoc. 2006, 42, 1537–1547. [Google Scholar] [CrossRef]
- Šimůnek, J.; Genuchten, M.T. Modeling Nonequilibrium Flow and Transport Processes Using HYDRUS. Vadose Zone J. 2008, 7, 782–797. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Bennett, J.M.; Biggs, A.A.J.; Marchuk, A.; Ghahramani, A. Assessing the Hydraulic Reduction Performance of HYDRUS-1D for Application of Alkaline Irrigation in Variably-Saturated Soils: Validation of PH Driven Hydraulic Reduction Scaling Factors. Agric. Water Manag. 2021, 256, 107101. [Google Scholar] [CrossRef]
- Blackmore, S.; Pedretti, D.; Mayer, K.U.; Smith, L.; Beckie, R.D. Evaluation of Single- and Dual-Porosity Models for Reproducing the Release of External and Internal Tracers from Heterogeneous Waste-Rock Piles. J. Contam. Hydrol. 2018, 214, 65–74. [Google Scholar] [CrossRef]
- Ma, D.; Shao, M. Simulating Infiltration into Stony Soils with a Dual-Porosity Model. Eur. J. Soil Sci. 2008, 59, 950–959. [Google Scholar] [CrossRef]
- Tremosa, J.; Debure, M.; Narayanasamy, S.; Redon, P.-O.; Jacques, D.; Claret, F.; Robinet, J.-C. Shale Weathering: A Lysimeter and Modelling Study for Flow, Transport, Gas Diffusion and Reactivity Assessment in the Critical Zone. J. Hydrol. 2020, 587, 124925. [Google Scholar] [CrossRef]
- Zhang, H.; Nordin, N.A.; Olson, M.S. Evaluating the Effects of Variable Water Chemistry on Bacterial Transport during Infiltration. J. Contam. Hydrol. 2013, 150, 54–64. [Google Scholar] [CrossRef]
- Astrup, T.; Mosbæk, H.; Christensen, T.H. Assessment of Long-Term Leaching from Waste Incineration Air-Pollution-Control Residues. Waste Manag. 2006, 26, 803–814. [Google Scholar] [CrossRef]
- Gonzalez, M.L.; Blanc, D.; de Brauer, C. Multi-Analytical Approach and Geochemical Modeling for Mineral Trace Element Speciation in MSWI Bottom-Ash. Waste Biomass Valor. 2019, 10, 547–560. [Google Scholar] [CrossRef]
- Xu, H.; Miao, J.; Chen, P.; Zhan, L.; Wang, Y. Chemical and Geotechnical Properties of Solidified/Stabilized MSWI Fly Ash Disposed at a Landfill in China. Eng. Geol. 2019, 255, 59–68. [Google Scholar] [CrossRef]
- Glasser, F.P. Fundamental Aspects of Cement Solidification and Stabilisation. J. Hazard. Mater. 1997, 52, 151–170. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Gao, M.; Guan, Y.; Wu, C.; Wang, Q.; Rao, Y.; Liu, S. Heavy Metal Leaching Behaviour and Long-Term Environmental Risk Assessment of Cement-Solidified Municipal Solid Waste Incineration Fly Ash in Sanitary Landfill. Chemosphere 2022, 300, 134571. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.; Xin, M.; Bian, R.; Wang, H.; Wang, Y.; Hu, Z.; Linh, H.N.; Zhang, D. Municipal Solid Waste Incineration Fly Ash Exposed to Carbonation and Acid Rain Corrosion Scenarios: Release Behavior, Environmental Risk, and Dissolution Mechanism of Toxic Metals. Sci. Total Environ. 2020, 744, 140857. [Google Scholar] [CrossRef]
- Garrabrants, A.C.; Sanchez, F.; Kosson, D.S. Changes in Constituent Equilibrium Leaching and Pore Water Characteristics of a Portland Cement Mortar as a Result of Carbonation. Waste Manag. 2004, 24, 19–36. [Google Scholar] [CrossRef]
- Ministry of the Environment Environmental Quality Standards for Soil Pollution. Available online: https://www.env.go.jp/en/water/soil/sp.html (accessed on 30 May 2023).
- Lager, T.; Delay, M.; Karius, V.; Hamer, K.; Frimmel, F.H.; Schulz, H.D. Determination and Quantification of the Release of Inorganic Contaminants from Municipal Waste Incineration Ash. Acta Hydrochim. Hydrobiol. 2006, 34, 73–85. [Google Scholar] [CrossRef]
- Dijkstra, J.J.; van der Sloot, H.A.; Comans, R.N.J. The Leaching of Major and Trace Elements from MSWI Bottom Ash as a Function of PH and Time. Appl. Geochem. 2006, 21, 335–351. [Google Scholar] [CrossRef]
- Hareeparsad, S.; Tiruta-Barna, L.; Brouckaert, C.J.; Buckley, C.A. Quantitative Geochemical Modelling Using Leaching Tests: Application for Coal Ashes Produced by Two South African Thermal Processes. J. Hazard. Mater. 2011, 186, 1163–1173. [Google Scholar] [CrossRef]
- Hyks, J.; Astrup, T.; Christensen, T.H. Influence of Test Conditions on Solubility Controlled Leaching Predictions from Air-Pollution-Control Residues. Waste Manag. Res. 2007, 25, 457–466. [Google Scholar] [CrossRef]
- Karamalidis, A.; Voudrias, E. Leaching and Immobilization Behavior of Zn and Cr from Cement-Based Stabilization/Solidification of Ash Produced from Incineration of Refinery Oily Sludge. Environ. Eng. Sci. 2009, 26, 81–96. [Google Scholar] [CrossRef]
- Meima, J.A.; Comans, R.N.J. Geochemical Modeling of Weathering Reactions in Municipal Solid Waste Incinerator Bottom Ash. Environ. Sci. Technol. 1997, 31, 1269–1276. [Google Scholar] [CrossRef]
- Yin, K.; Chan, W.P.; Dou, X.; Ren, F.; Chang, V.W.-C. Measurements, Factor Analysis and Modeling of Element Leaching from Incineration Bottom Ashes for Quantitative Component Effects. J. Clean. Prod. 2017, 165, 477–490. [Google Scholar] [CrossRef]
- Wang, P.; Chen, X.; Zeng, G.; Dong, Z.; Liu, S.; Zhang, X.; Wang, C. Long-Term Performance of Cement-Stabilized/Solidified Pb-Contaminated Soil under Simulated Erosive Environments. Water 2022, 14, 3314. [Google Scholar] [CrossRef]
- Ahmed, M.; Matsumoto, M.; Ozaki, A.; Thinh, N.V.; Kurosawa, K. Heavy Metal Contamination of Irrigation Water, Soil, and Vegetables and the Difference between Dry and Wet Seasons Near a Multi-Industry Zone in Bangladesh. Water 2019, 11, 583. [Google Scholar] [CrossRef] [Green Version]
- Boum-Nkot, S.N.; Nlend, B.; Komba, D.; Ndondo, G.R.N.; Bello, M.; Fongoh, E.J.; Ntamak-Nida, M.-J.; Etame, J. Hydrochemistry and Assessment of Heavy Metals Groundwater Contamination in an Industrialized City of Sub-Saharan Africa (Douala, Cameroon). Implication on Human Health. HydroResearch 2023, 6, 52–64. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Chandrajith, R.; Janardhana Raju, N.; Antunes, I.M.H.R. Provincial and Seasonal Influences on Heavy Metals in the Noyyal River of South India and Their Human Health Hazards. Environ. Res. 2022, 204, 111998. [Google Scholar] [CrossRef]
- Dijkstra, J.J.; Meeussen, J.C.L.; Van der Sloot, H.A.; Comans, R.N.J. A Consistent Geochemical Modelling Approach for the Leaching and Reactive Transport of Major and Trace Elements in MSWI Bottom Ash. Appl. Geochem. 2008, 23, 1544–1562. [Google Scholar] [CrossRef] [Green Version]
- Quina, M.J.; Bordado, J.C.M.; Quinta-Ferreira, R.M. The Influence of PH on the Leaching Behaviour of Inorganic Components from Municipal Solid Waste APC Residues. Waste Manag. 2009, 29, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Baciocchi, R.; Polettini, A.; Pomi, R.; Hills, C.D.; Carey, P.J. Current Status and Perspectives of Accelerated Carbonation Processes on Municipal Waste Combustion Residues. Environ. Monit. Assess. 2007, 135, 55–75. [Google Scholar] [CrossRef]
- Van der Sloot, H.A. Characterization of the Leaching Behaviour of Concrete Mortars and of Cement–Stabilized Wastes with Different Waste Loading for Long Term Environmental Assessment. Waste Manag. 2002, 22, 181–186. [Google Scholar] [CrossRef] [PubMed]






| Phase | Water Content and Retention Characteristics | Hydraulic Conductivity | Water and Mass Transfer | |||||
|---|---|---|---|---|---|---|---|---|
| θr[-] | θs [-] | α [1/cm] | n [-] | Ks [cm/year] | L [-] | Omega [1/year] | Gs [T1] | |
| Immobile | 0 | 0.162 | 0.03 | 2.0563 | 16,277 | 1 | 1 | 1 |
| Mobile | 0 | 0.378 | ||||||
| Mineral Phase | Chemical Formula | SI | Concentration (mmol/cm3) |
|---|---|---|---|
| Halite | NaCl | −7.39 | 2.9 × 10−2 |
| Sylvite | KCl | −6.21 | 7.9 × 10−3 |
| Gypsum | CaSO4∙2H2O | −0.91 | 8.2 × 10−3 |
| Calcite | CaCO3 | −0.89 | 1.9 × 10−1 |
| Portlandite | Ca(OH)2 | −3.34 | 9.8 × 10−3 |
| Ettringite | Ca₆Al₂(SO₄)₃(OH)₁₂·26H₂O | −8.89 | 1.0 |
| Antarcticite | CaCl2:6H2O | −11.81 | 2.2 × 10−6 |
| K-Feldspar | KAlSi3O8 | 1.14 | 3.3 × 10−1 |
| Element | BFA | SFA-F | SFA-O | SFA-D | SFA-N |
|---|---|---|---|---|---|
| Ca | 248,000 | 160,000 | 136,000 | 136,000 | 158,000 |
| Na | 260 | 98.0 | 55.0 | 60.0 | 60.0 |
| K | 4000 | 2250 | 2500 | 1870 | 1730 |
| Mg | 11,000 | 9300 | 10,500 | 9970 | 10,600 |
| Pb | 51.4 | 40.6 | 38.1 | 36.2 | 35.0 |
| As | 7.44 | 6.43 | 6.75 | 6.35 | 6.33 |
| T-Cr | 71.4 | 51.2 | 30.1 | 28.8 | 26.1 |
| Cd | 1.78 | 2.91 | 1.41 | 1.32 | 1.24 |
| Cu | 480 | 200 | 190 | 180 | 160 |
| Ni | 50.0 | 37.0 | 34.0 | 30.0 | 30.0 |
| Zn | 24,900 | 8320 | 9980 | 9000 | 5500 |
| Sample | Pb (mg/L) | T-Cr (mg/L) | As (mg/L) | Cd (mg/L) |
|---|---|---|---|---|
| BFA | 0.0141 | 0.0311 | <0.001 a | <0.001 a |
| SFA-F | 0.00304 | 0.108 | <0.001 a | <0.001 a |
| SFA-O | <0.001 a | 0.0300 | <0.001 a | <0.001 a |
| SFA-D | <0.001 a | 0.0240 | <0.001 a | <0.001 a |
| SFA-N | <0.001 a | 0.0413 | <0.001 a | <0.001 a |
| Limit values | 0.01 | 0.05 b | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, J.R.; Sekito, T.; Dote, Y. Long-Term Leaching Behavior and Geochemical Modeling of Cement Solidified Incineration Fly Ash Containing Waste Tires and Wood Biomass. Minerals 2023, 13, 823. https://doi.org/10.3390/min13060823
Santiago JR, Sekito T, Dote Y. Long-Term Leaching Behavior and Geochemical Modeling of Cement Solidified Incineration Fly Ash Containing Waste Tires and Wood Biomass. Minerals. 2023; 13(6):823. https://doi.org/10.3390/min13060823
Chicago/Turabian StyleSantiago, Jose Rodolfo, Tomoo Sekito, and Yutaka Dote. 2023. "Long-Term Leaching Behavior and Geochemical Modeling of Cement Solidified Incineration Fly Ash Containing Waste Tires and Wood Biomass" Minerals 13, no. 6: 823. https://doi.org/10.3390/min13060823