Antibacterial Activity of Four Plant Extracts Extracted from Traditional Chinese Medicinal Plants against Listeria monocytogenes, Escherichia coli, and Salmonella enterica subsp. enterica serovar Enteritidis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Reference Strains and Clinical Isolates
2.2. Preparation of Plant Extracts
2.3. Determination of Antibacterial Activity In Vitro
2.4. Determination of Bactericidal Activity in an In Vitro Cecum Model
2.5. Statistical Analysis
3. Results
3.1. Antibacterial Activity In Vitro
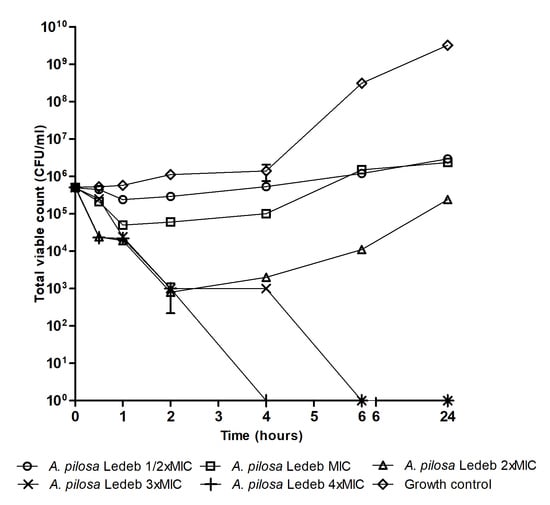
3.2. Bactericidal Activity in an In Vitro Cecum Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. Antimicrobial Resistance Global Report on Surveillance 2014; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Choski, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar]
- Muaz, K.; Riaz, M.; Akhtar, S.; Park, S.; Ismail, A. Antibiotic Residues in Chicken Meat: Global Prevalence, Threats, and Decontamination Strategies: A Review. J. Food Prot. 2018, 81, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.J.; Richards, J.D. Antibiotic Growth Promoters in Agriculture: History and Mode of Action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Mehdi, Y.; Létourneau-Montminy, M.P.; Gaucher, M.L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of Antibiotics in Broiler Production: Global Impacts and Alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Chopra, I.; Marilyn, R. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Silver, L.L.; Bostian, K.A. Minireview Discovery and Development of New Antibiotics: The Problem of Antibiotic Resistance. Antimicrob. Agents Chemother. 1993, 37, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Umaraw, P.; Prajapati, A.; Verma, A.K.; Pathak, V.; Singh, V.P. Control of Campylobacter in Poultry Industry from Farm to Poultry Processing Unit: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 659–665. [Google Scholar] [CrossRef]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Grande, H.; Weaver, B.; Papp, K.; Horwinski, J.; Koch, B.; Hungate, B.A.; Liu, C.M.; et al. Antibiotic-Resistant Escherichia Coli from Retail Poultry Meat with Different Antibiotic Use Claims. BMC Microbiol. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Van Galen, E. Traditional Herbal Medicines Worldwide, from Reappraisal to Assessment in Europe. J. Ethnopharmacol. 2014, 158, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Bensky, D.; Clavey, S.; Stoger, E.; Gamble, A.; Bensky, L.L. Chinese Herbal Medicine Materia Medica, 3rd ed.; Eastland Press: Seattle, WA, USA, 2004. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as Antibiotic Alternatives to Promote Growth and Enhance Host Health. Vet. Res. 2018, 49, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitemerere, T.A.; Mukanganyama, S. Evaluation of Cell Membrane Integrity as a Potential Antimicrobial Target for Plant Products. BMC Complement. Altern. Med. 2014, 14, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi Gheisar, M.; Kim, I.H. Phytobiotics in Poultry and Swine Nutrition—A Review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar] [CrossRef]
- Dhami, D.S.; Shah, G.C.; Kumar, V.; Joshi, Y.; Tripathi, M.; Bisht, M. Essential Oil Composition and Antibacterial Activity of Agrimonia Pilosa Ledeb (Rosaceae). Chem. Sci. Trans. 2018, 7, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Joung, D.K.; Mun, S.H.; Lee, K.S.; Kang, O.H.; Choi, J.G.; Kim, S.B.; Gong, R.; Chong, M.S.; Kim, Y.C.; Lee, D.S.; et al. The Antibacterial Assay of Tectorigenin with Detergents or ATPase Inhibitors Against Methicillin-Resistant Staphylococcus Aureus. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Liu, J.; Yang, L.; Li, X. Study on the Optimization of Extraction Technology of Anemonin from Pulsatilla Chinensis and Its Inhibitory Effect on Alternaria Panax. J. Dis. Med. Plants 2019, 5, 94–102. [Google Scholar] [CrossRef]
- Hua, S.; Zhang, Y.; Liu, J.; Dong, L.; Huang, J.; Lin, D.; Fu, X. Ethnomedicine, Phytochemistry and Pharmacology of Smilax Glabra: An Important Traditional Chinese Medicine. Am. J. Chin. Med. 2018, 46, 261–297. [Google Scholar] [CrossRef]
- Fong, S.C.; Mulyana, Y.; Girawan, D. Antibacterial Effect of Pulsatilla Chinensis towards Staphylococcus Aureus, Shigella Dysenteriae, and Salmonella Typhi. Althea Med. J. 2016, 3, 292–295. [Google Scholar] [CrossRef] [Green Version]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Plants for a Future. Plants for a Future: Belamcanda Chinensis - (L.) DC. Available online: https://pfaf.org/user/Plant.aspx?LatinName=Belamcanda+chinensis (accessed on 3 May 2020).
- Plants for a Future. Plants for a Future: Agrimonia Pilosa – Ledeb. Available online: https://pfaf.org/user/Plant.aspx?LatinName=Agrimonia+pilosa (accessed on 3 May 2020).
- Plants for a Future. Plants for a Future: Pulsatilla Chinensis - (Bunge.)Regel. Available online: https://pfaf.org/user/Plant.aspx?LatinName=Pulsatilla+chinensis (accessed on 3 May 2020).
- Plants for a Future. Plants for a Future: Smilax China C- L. Available online: https://pfaf.org/user/plant.aspx?latinname=Smilax+china (accessed on 3 May 2020).
- Bioline. Available online: https://www.bioline.com/uk/downloads/dl/file/id/2697/mytaq_red_mix_product_manual.pdf (accessed on 16 April 2020).
- Principi, N.; Esposito, S. Antibiotic Administration and the Development of Obesity in Children. Int. J. Antimicrob. Agents 2016, 47, 171–177. [Google Scholar] [CrossRef]
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 24 January 2020).
- Clinical and Laboratory Standards Institute. Methods for Dilution: Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 8th ed.; CLSI: Wayne, PA, USA, 2009; Volume 29. [Google Scholar]
- Johny, A.K.; Darre, M.J.; Donoghue, A.M.; Donoghue, D.J.; Venkitanarayanan, K. Antibacterial Effect of Trans-Cinnamaldehyde, Eugenol, Carvacrol, and Thymol on Salmonella Enteritidis and Campylobacter Jejuni in Chicken Cecal Contents in Vitro. J. Appl. Poult. Res. 2010, 19, 237–244. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; CLSI: Wayne, PA, USA, 2009; Volume 19. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 8.1. 2018. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf (accessed on 3 May 2020).
- Franzblau, S.G.; Cross, C. Comparative in Vitro Antimicrobial Activity of Chinese Medicinal Herbs. J. Ethnopharmacol. 1986, 15, 279–288. [Google Scholar] [CrossRef]
- Kasai, S.; Watanabe, S.; Kawabata, J.; Tahara, S.; Mizuntani, J. Antimicrobial Catechin Derivatives of Agrimonia Pilosa. Phytochemistry 1992, 31, 787–789. [Google Scholar] [CrossRef]
- Xu, S.; Shang, M.; Liu, G.; Xu, F.; Wang, X.; Shou, C.; Cai, S. Chemical Constituents from the Rhizomes of Smilax Glabra and Their Antimicrobial Activity. Molecules 2013, 18, 5265–5287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ríos, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, M.; Kashihara, M.; Ishiguro, K.; Takagi, S. Antimicrobial Principles of Xian He Cao (Agrimonia Pilosa). Planta Med. 1989, 55, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Skroza, D.; Šimat, V.; Smole Možina, S.; Katalinić, V.; Boban, N.; Generalić Mekinić, I. Interactions of Resveratrol with Other Phenolics and Activity against Food-Borne Pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Kurahachi, M.; Nonaka, A.; Miyamoto, T. A Study of the Antibacterial Mechanism of Catechins: Isolation and Identification of Escherichia Coli Cell Surface Proteins That Interact with Epigallocatechin Gallate. Food Control. 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Diarra, M.S.; Hassan, Y.I.; Block, G.S.; Drover, J.C.G.; Delaquis, P.; Oomah, B.D. Antibacterial Activities of a Polyphenolic-Rich Extract Prepared from American Cranberry (Vaccinium Macrocarpon) Fruit Pomace Against Listeria Spp. LWT - Food Sci. Technol. 2020, 123. [Google Scholar] [CrossRef]
- Kumar, S.; Madaan, R.; Farooq, A.; Sharma, A. Plant Review The Genus Pulsatilla: A Review. Pharmacogn. Rev. 2008, 2, 116–124. [Google Scholar]
- Khan, M.I.; Ahhmed, A.; Shin, J.H.; Baek, J.S.; Kim, M.Y.; Kim, J.D. Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram Positive and Gram Negative Bacteria, a Comprehensive Study in Vitro and in Vivo. Evid. Based Complement. Alternat. Med. 2018, 2018, 3486106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.K.; Jung, B.S.; Na, D.S.; Yu, H.H.; Kim, J.S.; Paik, H.D. The Impact of Antimicrobial Effect of Chestnut Inner Shell Extracts against Campylobacter Jejuni in Chicken Meat. LWT - Food Sci. Technol. 2016, 65, 746–750. [Google Scholar] [CrossRef]



| Species | Isolate | Source |
|---|---|---|
| Listeria monocytogenes | NCTC 11994 | Reference Queen’s University Belfast (QUB) |
| LS12519 | Retail ready to eat sliced meat Agri-Food and Biosciences Institute (AFBI) | |
| OT11230 | Retail chopping board (AFBI) | |
| CP102 | Retail ham and cheese filling (AFBI) | |
| CP1132 | Retail cooked chicken breast (AFBI) | |
| QA1018 | Quality assurance sample (AFBI) | |
| Salmonella enteritidis | NCTC 0074 | Reference (QUB) |
| 1F6144 | Quality assurance sample (AFBI) | |
| LE103 | Egg filter (AFBI) | |
| QA04/19 | Quality assurance sample (AFBI) | |
| Escherichia coli | ATCC 25922 | Reference (QUB) |
| UM004 | Urinary tract infection (QUB) | |
| UM011 | Urinary tract infection (QUB) | |
| UM012 | Urinary tract infection (QUB) |
| Pathogen | Plant Extract | |||||
|---|---|---|---|---|---|---|
| Agrimonia pilosa Ledeb | Smilax glabra Roxb | Anemone chinensis Bunge | Iris domestica (L.) Goldblatt and Mabb | Ampicillin | ||
| L. monocytogenes | NCTC 11994 | 31.25 | 62.5 | 125 | 125 | 0.25 |
| LS12519 | 31.25 | 31.25 | 125 | 125 | 0.5 | |
| OT11230 | 62.5 | 125 | 125 | 125 | 0.5 | |
| CP102 | 31.25 | 31.25 | 125 | 125 | 0.25 | |
| CP1132 | 125 | 31.25 | 125 | 125 | 0.25 | |
| QA1018 | 31.25 | 31.25 | 125 | 125 | 1 | |
| S. enteritidis | NCTC 0074 | 500 | 250 | 62.5 | 250 | 4 |
| IF6144 | 125 | 125 | 62.5 | 125 | 2 | |
| LE103 | 125 | 125 | 62.5 | 125 | 4 | |
| QA0419 | 125 | 125 | 62.5 | 125 | 8 | |
| E. coli | ATCC 25922 | 7.81 | 125 | 125 | 62.5 | 8 |
| UM004 | 7.81 | 125 | 125 | 62.5 | 4 | |
| UM011 | 7.81 | 125 | 125 | 62.5 | 4 | |
| UM012 | 7.81 | 125 | 125 | 62.5 | 4 | |
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Time (Hours) | A. pilosa Ledeb | A. chinensis Bunge | S. glabra Roxb | I. domestica (L.) Goldblatt and Mabb | Ampicillin | SEM | p |
| 0 | 0 | 0 | 0 | 0 | 0 | 0.000 | NS |
| 0.5 | 96.24 b | 0.76 a | 0.25 a | 1.92 a | 95.10 b | 0.940 | < 0.001 |
| 1 | 96.16 b | 0.76 a | 1.49 a | 2.31 a | 95.92 b | 0.890 | < 0.001 |
| 2 | 99.60 b | 99.24 b | 99.27 b | 31.01 a | 99.66 b | 0.278 | < 0.001 |
| 4 | 99.99 c | 99.62 b | 99.99 c | 56.59 a | 99.99 c | 0.480 | < 0.001 |
| 6 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 0.000 | NS |
| 24 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 0.000 | NS |
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Time (Hours) | A. pilosa Ledeb | A. chinensis Bunge | S. glabra Roxb | I. domestica (L.) Goldblatt and Mabb | Ampicillin | SEM | p |
| 0 | 0 | 0 | 0 | 0 | 0 | 0.000 | NS |
| 0.5 | 95.47 c | 96.27 c | 93.64 b | 47.81 a | 95.97 c | 1.019 | < 0.001 |
| 1 | 95.67 c | 99.63 d | 95.65 c | 43.83 a | 96.47 b | 0.358 | < 0.001 |
| 2 | 99.80 b | 99.63 b | 99.61 b | 44.23 a | 99.62 b | 0.483 | < 0.001 |
| 4 | 99.99 b | 99.99 b | 99.80 b | 42.63 a | 99.62 b | 0.410 | < 0.001 |
| 6 | 99.99 b | 99.99 b | 99.99 b | 99.18 a | 99.99 b | 0.020 | < 0.001 |
| 24 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 0.000 | NS |
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Time (Hours) | A. pilosa Ledeb | A. chinensis Bunge | S. glabra Roxb | I. domestica (L.) Goldblatt and Mabb | Ampicillin | SEM | p |
| 0 | 0 | 0 | 0 | 0 | 0 | 0.000 | NS |
| 0.5 | 1.18 a | 2.26 a | 1.15 a | 0.77 a | 95.75 b | 2.559 | < 0.001 |
| 1 | 1.58 a | 3.02 a | 2.32 a | 0.77 a | 96.03 b | 2.174 | < 0.001 |
| 2 | 99.13 b | 99.25 b | 99.22 b | 33.59 a | 99.60 b | 0.761 | < 0.001 |
| 4 | 99.29 b | 99.62 b | 99.22 b | 61.25 a | 99.80 b | 2.692 | < 0.001 |
| 6 | 99.60 b | 99.99 c | 99.22 a | 99.99 c | 99.99 c | 0.132 | < 0.001 |
| 24 | 99.52 b | 99.99c | 99.61a | 99.99 c | 99.99 c | 0.206 | < 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMurray, R.L.; Ball, M.E.E.; Tunney, M.M.; Corcionivoschi, N.; Situ, C. Antibacterial Activity of Four Plant Extracts Extracted from Traditional Chinese Medicinal Plants against Listeria monocytogenes, Escherichia coli, and Salmonella enterica subsp. enterica serovar Enteritidis. Microorganisms 2020, 8, 962. https://doi.org/10.3390/microorganisms8060962
McMurray RL, Ball MEE, Tunney MM, Corcionivoschi N, Situ C. Antibacterial Activity of Four Plant Extracts Extracted from Traditional Chinese Medicinal Plants against Listeria monocytogenes, Escherichia coli, and Salmonella enterica subsp. enterica serovar Enteritidis. Microorganisms. 2020; 8(6):962. https://doi.org/10.3390/microorganisms8060962
Chicago/Turabian StyleMcMurray, R.L., M.E.E. Ball, M.M. Tunney, N. Corcionivoschi, and C. Situ. 2020. "Antibacterial Activity of Four Plant Extracts Extracted from Traditional Chinese Medicinal Plants against Listeria monocytogenes, Escherichia coli, and Salmonella enterica subsp. enterica serovar Enteritidis" Microorganisms 8, no. 6: 962. https://doi.org/10.3390/microorganisms8060962