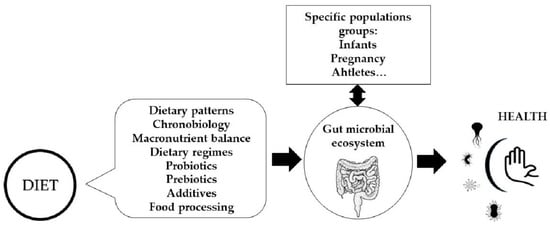
The Influence of Dietary Factors on the Gut Microbiota
Abstract
:1. Introduction
2. Dietary Regimes, Chronobiology, and the Gut Microbiota
3. Macronutrients–Microbiota Interactions
4. Food Processing/Cooking and Gut Microbiota
5. Food Additives and Gut Microbiota
6. Dietary Pattern in Particular Physiological Conditions and the Microbiota
6.1. Infant Feeding and Microbiota Development
6.2. Pregnancy
6.3. Exercise
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Boscaini, S.; Leigh, S.-J.; Lavelle, A.; García-Cabrerizo, R.; Lipuma, T.; Clarke, G.; Schellekens, H.; Cryan, J.F. Microbiota and Body Weight Control: Weight Watchers Within? Mol. Metab. 2022, 57, 101427. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetissov, S.O. Role of the Gut Microbiota in Host Appetite Control: Bacterial Growth to Animal Feeding Behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Romaní-Pérez, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Liébana-García, R.; Olivares, M.; Sanz, Y. The Microbiota and the Gut-Brain Axis in Controlling Food Intake and Energy Homeostasis. Int. J. Mol. Sci. 2021, 22, 5830. [Google Scholar] [CrossRef]
- Van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, K.; Kamada, N. Diet-Microbiota Interactions in Inflammatory Bowel Disease. Nutrients 2021, 13, 1533. [Google Scholar] [CrossRef]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 3183. [Google Scholar] [CrossRef]
- Moles, L.; Otaegui, D. The Impact of Diet on Microbiota Evolution and Human Health. Is Diet an Adequate Tool for Microbiota Modulation? Nutrients 2020, 12, 1654. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-Microbiota Interactions and Personalized Nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klassen, L.; Xing, X.; Tingley, J.P.; Low, K.E.; King, M.L.; Reintjes, G.; Abbott, D.W. Approaches to Investigate Selective Dietary Polysaccharide Utilization by Human Gut Microbiota at a Functional Level. Front. Microbiol. 2021, 12, 632684. [Google Scholar] [CrossRef] [PubMed]
- Bailén, M.; Tabone, M.; Bressa, C.; Lominchar, M.G.M.; Larrosa, M.; González-Soltero, R. Unraveling Gut Microbiota Signatures Associated with PPARD and PARGC1A Genetic Polymorphisms in a Healthy Population. Genes 2022, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ratiner, K.; Elinav, E. Circadian Influences of Diet on the Microbiome and Immunity. Trends Immunol. 2020, 41, 512–530. [Google Scholar] [CrossRef]
- Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian Rhythms from Flies to Human. Nature 2002, 417, 329–335. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Musaad, S.M.; Holscher, H.D. Time of Day and Eating Behaviors Are Associated with the Composition and Function of the Human Gastrointestinal Microbiota. Am. J. Clin. Nutr. 2017, 106, 1220–1231. [Google Scholar] [CrossRef] [Green Version]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef] [Green Version]
- Nobs, S.P.; Tuganbaev, T.; Elinav, E. Microbiome Diurnal Rhythmicity and Its Impact on Host Physiology and Disease Risk. EMBO Rep. 2019, 20, e47129. [Google Scholar] [CrossRef]
- Galanakis, C.M. Dietary Fiber: Properties, Recovery, and Applications, 1st ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e4. [Google Scholar] [CrossRef] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Montalvo Lominchar, M.G.; San Juan, C.; Larrosa, M. Microbiota Features Associated with a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Mohanty, A. Gut Microbiota and Covid-19-Possible Link and Implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Tajbakhsh, A.; Gheibihayat, S.M.; Moattari, A.; Razban, V.; Berenjian, A.; Savardashtaki, A.; Negahdaripour, M. Probiotics/Prebiotics in Viral Respiratory Infections: Implication for Emerging Pathogens. Recent Pat. Biotechnol. 2021, 15, 112–136. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic Inulin-Type Fructans Induce Specific Changes in the Human Gut Microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef]
- Newnham, E.D. Coeliac Disease in the 21st Century: Paradigm Shifts in the Modern Age. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 82–85. [Google Scholar] [CrossRef] [Green Version]
- Cândido, F.G.; Valente, F.X.; Grześkowiak, Ł.M.; Moreira, A.P.B.; Rocha, D.M.U.P.; Alfenas, R.d.C.G. Impact of Dietary Fat on Gut Microbiota and Low-Grade Systemic Inflammation: Mechanisms and Clinical Implications on Obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural Resilience of the Gut Microbiota in Adult Mice under High-Fat Dietary Perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary Fat, the Gut Microbiota, and Metabolic Health—A Systematic Review Conducted within the MyNewGut Project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of Dietary Fat on Gut Microbiota and Faecal Metabolites, and Their Relationship with Cardiometabolic Risk Factors: A 6-Month Randomised Controlled-Feeding Trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [Green Version]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic Diet Enhances Neurovascular Function with Altered Gut Microbiome in Young Healthy Mice. Sci. Rep. 2018, 8, 6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A Randomised Trial of the Effect of Omega-3 Polyunsaturated Fatty Acid Supplements on the Human Intestinal Microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Reidelberger, R.D.; Chelikani, P.K. Inulin Fiber Dose-Dependently Modulates Energy Balance, Glucose Tolerance, Gut Microbiota, Hormones and Diet Preference in High-Fat-Fed Male Rats. J. Nutr. Biochem. 2018, 59, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Do Rosário, D.K.A.; da Silva Mutz, Y.; Peixoto, J.M.C.; Oliveira, S.B.S.; de Carvalho, R.V.; Carneiro, J.C.S.; de São José, J.F.B.; Bernardes, P.C. Ultrasound Improves Chemical Reduction of Natural Contaminant Microbiota and Salmonella enterica Subsp. enterica on Strawberries. Int. J. Food Microbiol. 2017, 241, 23–29. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-Regulating the Human Intestinal Microbiome Using Whole Plant Foods, Polyphenols, and/or Fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef]
- Gutiérrez-Díaz, I.; Salazar, N.; Pérez-Jiménez, J.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. New Players in the Relationship between Diet and Microbiota: The Role of Macromolecular Antioxidant Polyphenols. Eur. J. Nutr. 2021, 60, 1403–1413. [Google Scholar] [CrossRef]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef]
- Dostal Webster, A.; Staley, C.; Hamilton, M.J.; Huang, M.; Fryxell, K.; Erickson, R.; Kabage, A.J.; Sadowsky, M.J.; Khoruts, A. Influence of Short-Term Changes in Dietary Sulfur on the Relative Abundances of Intestinal Sulfate-Reducing Bacteria. Gut Microbes 2019, 10, 447–457. [Google Scholar] [CrossRef]
- Hori, T.; Matsuda, K.; Oishi, K. Probiotics: A Dietary Factor to Modulate the Gut Microbiome, Host Immune System, and Gut-Brain Interaction. Microorganisms 2020, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Pallagatti, S.; Kalucha, A.; Kaur, H. Probiotics. Going on the natural way. J. Clin. Exp. Dent. 2011, 3, e150–e154. [Google Scholar] [CrossRef]
- Darwazeh, A. Probiotics and oral disease: An update. Smile Dent. J. 2011, 6, 6–8. [Google Scholar]
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2020, 17, 2647. [Google Scholar] [CrossRef] [Green Version]
- Mörkl, S.M.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar]
- Day, A.W.; Kumamoto, C.A. Gut Microbiome Dysbiosis in Alcoholism: Consequences for Health and Recovery. Front. Cell. Infect. Microbiol. 2022, 12, 840164. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.E. Impact of drinking alcohol on gut microbiota: Recent perspectives on ethanol and alcoholic beverage. Curr. Opin. Food Sci. 2021, 37, 91–97. [Google Scholar] [CrossRef]
- Llopis, M.; Cassard, A.M.; Wrzosek, L.; Boschat, L.; Bruneau, A.; Ferrere, G.; Puchois, V.; Martin, J.C.; Lepage, P.; Le Roy, T.; et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016, 65, 830–839. [Google Scholar] [CrossRef]
- González-Zancada, N.; Redondo-Useros, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A.; Nova, E. Association of Moderate Beer Consumption with the Gut Microbiota and SCFA of Healthy Adults. Molecules 2020, 25, 4772. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, Y.; Fang, C.; Wijffels, R.H.; Xu, Y. Can We Control Microbiota in Spontaneous Food Fermentation?—Chinese Liquor as a Case Example. Trends Food Sci. Technol. 2021, 110, 321–331. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [Green Version]
- Carmody, R.N.; Bisanz, J.E.; Bowen, B.P.; Maurice, C.F.; Lyalina, S.; Louie, K.B.; Treen, D.; Chadaideh, K.S.; Maini Rekdal, V.; Bess, E.N.; et al. Cooking Shapes the Structure and Function of the Gut Microbiome. Nat. Microbiol. 2019, 4, 2052–2063. [Google Scholar] [CrossRef]
- Aljahdali, N.; Gadonna-Widehem, P.; Anton, P.M.; Carbonero, F. Gut Microbiota Modulation by Dietary Barley Malt Melanoidins. Nutrients 2020, 12, 241. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Chen, Y.A.; Tuohy, K.M. A Comparative in Vitro Investigation into the Effects of Cooked Meats on the Human Faecal Microbiota. Anaerobe 2010, 16, 572–577. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Jiménez-Hernández, N.; D’Auria, G.; Francino, M.P.; Rufián-Henares, J.A. Effect of Food Thermal Processing on the Composition of the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 11500–11509. [Google Scholar] [CrossRef]
- Yu, X.; Zuo, T. Editorial: Food Additives, Cooking and Processing: Impact on the Microbiome. Front. Nutr. 2021, 8, 731040. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Miclotte, L.; Van de Wiele, T. Food Processing, Gut Microbiota and the Globesity Problem. Crit. Rev. Food Sci. Nutr. 2020, 60, 1769–1782. [Google Scholar] [CrossRef]
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of Food Additives on Gut Homeostasis. Nutrients 2019, 11, 2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abiega-Franyutti, P.; Freyre-Fonseca, V. Chronic Consumption of Food-Additives Lead to Changes via Microbiota Gut-Brain Axis. Toxicology 2021, 464, 153001. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High Salt Diet Exacerbates Colitis in Mice by Decreasing Lactobacillus Levels and Butyrate Production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Raoul, P.; Cintoni, M.; Palombaro, M.; Basso, L.; Rinninella, E.; Gasbarrini, A.; Mele, M.C. Food Additives, a Key Environmental Factor in the Development of IBD through Gut Dysbiosis. Microorganisms 2022, 13, 167. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Del Pozo, S.; Gómez-Martínez, S.; Díaz, L.E.; Nova, E.; Urrialde, R.; Marcos, A. Potential Effects of Sucralose and Saccharin on Gut Microbiota: A Review. Nutrients 2022, 14, 1682. [Google Scholar] [CrossRef]
- Le Huërou-Luron, I.; Blat, S.; Boudry, G.B.V. Formula-Feeding: Impacts on the Digestive Tract and Immediate and Long-Term Health Effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Vacca, M.; Raspini, B.; Calabrese, F.M.; Porri, D.; De Giuseppe, R.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; et al. The Establishment of the Gut Microbiota in 1-Year-Aged Infants: From Birth to Family Food. Eur. J. Nutr. 2022. [Google Scholar] [CrossRef]
- Conta, G.; Del Chierico, F.; Reddel, S.; Marini, F.; Sciubba, F.; Capuani, G.; Tomassini, A.; Di Cocco, M.E.; Laforgia, N.; Baldassarre, M.E.; et al. Longitudinal Multi-Omics Study of a Mother-Infant Dyad from Breastfeeding to Weaning: An Individualized Approach to Understand the Interactions Among Diet, Fecal Metabolome and Microbiota Composition. Front. Mol. Biosci. 2021, 8, 688440. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Tidjani Alou, M.; Khelaifia, S.; Bachar, D.; Lagier, J.-C.; Dione, N.; Brah, S.; Hugon, P.; Lombard, V.; Armougom, F.; et al. Increased Gut Redox and Depletion of Anaerobic and Methanogenic Prokaryotes in Severe Acute Malnutrition. Sci. Rep. 2016, 6, 26051. [Google Scholar] [CrossRef] [PubMed]
- Pekmez, C.T.; Dragsted, L.O.; Brahe, L.K. Gut Microbiota Alterations and Dietary Modulation in Childhood Malnutrition—The Role of Short Chain Fatty Acids. Clin. Nutr. 2019, 38, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.Z.; Al-Rumaihi, S.; Al-Absi, R.S.; Farah, H.; Elamin, M.; Nader, R.; Bouabidi, S.; Suleiman, S.E.; Nasr, S.; Al-Asmakh, M. Physiological Changes and Interactions Between Microbiome and the Host During Pregnancy. Front. Cell. Infect. Microbiol. 2022, 12, 824925. [Google Scholar] [CrossRef] [PubMed]
- Dreisbach, C.; Morgan, H.; Cochran, C.; Gyamfi, A.; Henderson, W.A.; Prescott, S. Metabolic and Microbial Changes Associated With Diet and Obesity During Pregnancy: What Can We Learn From Animal Studies? Front. Cell. Infect. Microbiol. 2021, 11, 795924. [Google Scholar] [CrossRef] [PubMed]
- Latino, C.; Gianatti, E.J.; Mehta, S.; Lo, J.; Devine, A.; Christophersen, C. Does a High Dietary Intake of Resistant Starch Affect Glycaemic Control and Alter the Gut Microbiome in Women with Gestational Diabetes? A Randomised Control Trial Protocol. BMC Pregnancy Childbirth 2022, 22, 46. [Google Scholar] [CrossRef]
- Miller, C.B.; Benny, P.; Riel, J.; Boushey, C.; Perez, R.; Khadka, V.; Qin, Y.; Maunakea, A.K.; Lee, M.-J. Adherence to Mediterranean Diet Impacts Gastrointestinal Microbial Diversity throughout Pregnancy. BMC Pregnancy Childbirth 2021, 21, 558. [Google Scholar] [CrossRef]
- Röytiö, H.; Mokkala, K.; Vahlberg, T.; Laitinen, K. Dietary Intake of Fat and Fibre According to Reference Values Relates to Higher Gut Microbiota Richness in Overweight Pregnant Women. Br. J. Nutr. 2017, 118, 343–352. [Google Scholar] [CrossRef]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between Physical Activity and Changes in Intestinal Microbiota Composition: A Systematic Review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef]
- Tzemah Shahar, R.; Koren, O.; Matarasso, S.; Shochat, T.; Magzal, F.; Agmon, M. Attributes of Physical Activity and Gut Microbiome in Adults: A Systematic Review. Int. J. Sports Med. 2020, 41, 801–814. [Google Scholar] [CrossRef]
- Han, M.; Yang, K.; Yang, P.; Zhong, C.; Chen, C.; Wang, S.; Lu, Q.; Ning, K. Stratification of Athletes’ Gut Microbiota: The Multifaceted Hubs Associated with Dietary Factors, Physical Characteristics and Performance. Gut Microbes 2020, 12, 1842991. [Google Scholar] [CrossRef] [PubMed]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.-G.; Choi, G.; Kim, S.-W.; Kim, B.-Y.; Lee, S.; Park, H. The Combination of Sport and Sport-Specific Diet Is Associated with Characteristics of Gut Microbiota: An Observational Study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murtaza, N.; Burke, L.M.; Vlahovich, N.; Charlesson, B.; O’Neill, H.M.; Ross, M.L.; Campbell, K.L.; Krause, L.; Morrison, M. Analysis of the Effects of Dietary Pattern on the Oral Microbiome of Elite Endurance Athletes. Nutrients 2019, 11, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nova, E.; Gómez-Martinez, S.; González-Soltero, R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms 2022, 10, 1368. https://doi.org/10.3390/microorganisms10071368
Nova E, Gómez-Martinez S, González-Soltero R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms. 2022; 10(7):1368. https://doi.org/10.3390/microorganisms10071368
Chicago/Turabian StyleNova, Esther, Sonia Gómez-Martinez, and Rocio González-Soltero. 2022. "The Influence of Dietary Factors on the Gut Microbiota" Microorganisms 10, no. 7: 1368. https://doi.org/10.3390/microorganisms10071368