Direct Growth of Carbon Nanotubes on New High-Density 3D Pyramid-Shaped Microelectrode Arrays for Brain-Machine Interfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Fabrication of Microelectrode Arrays (MEAs)
2.2. Shank Insulation
2.3. Masking Process
2.4. Tip Metallization and Lift-off
2.5. CNTs Growth
3. Electrochemical Measurements of the MEAs
3.1. Electrochemical Impedance Spectroscopy (EIS) and Cyclic Voltammetry (CV)
3.2. Charge Delivery Capacity (QCDC) and Charge Injection Capacity (Qinj)
3.3. Equivalent Circuit Model
4. Results
4.1. Characterization of the 3D MEAs
4.2. Masking Process
4.3. Tip-Metal Deposition
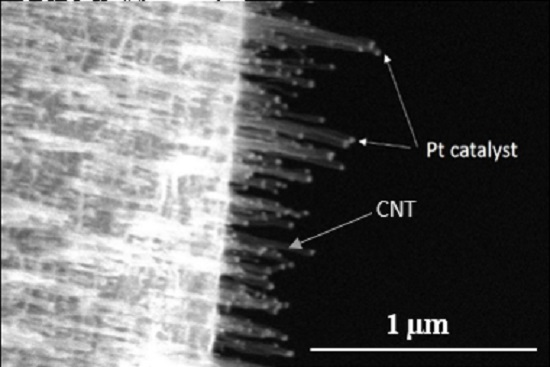
4.4. Tip-CNT Growth
4.5. Electrochemical Properties of the MEAs
4.6. Equivalent Electrode-Electrolyte Interface Circuit Model
5. Discussions
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coulombe, J.; Sawan, M.; Gervais, J.F. A highly flexible system for microstimulation of the visual cortex: Design and implementation. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Tolia, A.; Hansberry, D.R.; Duffis, E.J.; Barrese, J.C.; Gandhi, C.E.; Prestigiacomo, C.J. Current differential diagnoses and treatment options of vascular occlusions presenting as bilateral thalamic infarcts: A review of the literature. J. Neurointerv. Surg. 2013, 5, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Wise, K.D. Silicon microsystems for neuroscience and neural prostheses. IEEE Eng. Med. Biol. Mag. 2005, 24, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Normann, R.A. Technology insight: Future neuroprosthetic therapies for disorders of the nervous system. Nat. Clin. Pract. Neurol. 2007, 3, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Ghane-Motlagh, B.; Sawan, M. Design and Implementation Challenges of Microelectrode Arrays: A Review. Mater. Sci. Appl. 2013, 4, 483–495. [Google Scholar]
- Chang, T.Y.; Yadav, V.G.; Leo, S.D.; Mohedas, A.; Rajalingam, B.; Chen, C.L.; Selvarasah, S.; Dokmeci, M.R.; Khademhosseini, A. Cell and protein compatibility of parylene-C surfaces. Langmuir 2007, 23, 11718–11725. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.M.; McIntosh, J.S.; Bak, M.J. Long-term implants of Parylene-C coated microelectrodes. Med. Biol. Eng. Comput. 1988, 26, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.; von Metzen, R.P.; Ruther, P.; Stieglitz, T. Characterization of parylene C as an encapsulation material for implanted neural prostheses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Voskerician, G.; Shive, M.S.; Shawgo, R.S.; von Recum, H.; Anderson, J.M.; Cima, M.J.; Langer, R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials 2003, 24, 1959–1967. [Google Scholar] [CrossRef]
- Brummer, S.B.; Robblee, L.S.; Hambrecht, F.T. Criteria for selecting electrodes for electrical stimulation: Theoretical and practical considerations. Ann. N. Y. Acad. Sci. 1983, 405, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Geddes, L.A.; Roeder, R. Criteria for the selection of materials for implanted electrodes. Ann. Biomed. Eng. 2003, 31, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Fox, M.S. On the molecular basis of high negative interference. Proc. Natl. Acad. Sci. USA 1974, 71, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fishman, H.A.; Dai, H.; Harris, J.S. Neural stimulation with a carbon nanotube microelectrode array. Nano Lett. 2006, 6, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Phely-Bobin, T.S.; Tiano, T.; Farrell, B.; Fooksa, R.; Robblee, L.; Edell, D.; Czerw, R. Carbon nanotube based electrodes for neuroprosthetic applications. Mater. Res. Soc. Symp. Proc. 2006, 926. [Google Scholar] [CrossRef]
- Bhandari, R.; Negi, S.; Solzbacher, F. Wafer-scale fabrication of penetrating neural microelectrode arrays. Biomed. Microdevices 2010, 12, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Negi, S.; Rieth, L.; Normann, R.A.; Solzbacher, F. A novel masking method for high aspect ratio penetrating microelectrode arrays. J. Micromech. Micromeng. 2009, 19, 3. [Google Scholar] [CrossRef]
- Green, R.A.; Williams, C.M.; Lovell, N.H.; Poole-Waren, L.A. Novel neural interface for implant electrodes: Improving electroactivity of polypyrrole through MWNT incorporation. J. Mater. Sci. Mater. Med. 2008, 19, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- David-Pur, M.; Bareket-Keren, L.; Beit-Yaakove, G.; Raz-Prag, D.; Hanein, Y. All-carbon-nanotube flexible multi-electrode array for neuronal recording and stimulation. Biomed. Microdevices 2014, 16, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Minnikanti, S.; Peixoto, N. Implantable electrodes with carbon nanotube coatings. In Carbon Nanotubes Applications on Electron Devices; InTech: Novo Mesto, Croatia, 2011. [Google Scholar]
- Ansaldo, A.; Castagnola, E.; Maggiolini, E.; Fadiga, L.; Ricci, D. Superior electrochemical performance of carbon nanotubes directly grown on sharp microelectrodes. ACS Nano 2011, 5, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Choi, S.H.; Lee, T.Y.; Yoo, J.B.; Park, C.Y.; Jung, T.; Yu, S.; Yi, W.; Han, I.T.; Kim, J.M. Growth characteristics of carbon nanotubes using platinum catalyst by plasma enhanced chemical vapor deposition. Diam. Relat. Mater. 2003, 12, 878–883. [Google Scholar] [CrossRef]
- Takagi, D.; Homma, Y.; Hibino, H.; Suzuki, S.; Kobayashi, Y. Single-walled carbon nanotube growth from highly activated metal nanoparticles. Nano Lett. 2006, 6, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, I.; Gabriel, G.; Rius, G.; Villa, R.; Perez-Murano, F.; Lora-Tamayo, E.; Godignon, P. Vertically aligned multi-walled carbon nanotube growth on platinum electrodes for bio-impedance applications. Microelectron. Eng. 2009, 86, 806–808. [Google Scholar] [CrossRef]
- Kondo, H.; Fukuoka, N.; Maruyama, T. Low temperature growth of single-walled carbon nanotubes from Pt catalysts under low ethanol pressure by alcohol gas source method. J. Nanotechnol. 2012, 2012, 690304. [Google Scholar] [CrossRef]
- Liu, X.; Guo, L.; Morris, D.; Kane, A.B.; Hurt, R.H. Targeted removal of bioavailable metal as a detoxification strategy for carbon nanotubes. Carbon 2008, 46, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Ghane-Motlagh, B.; Sawan, M. High-Density 3D Pyramid-Shaped Microelectrode Arrays for Brain-Machine Interface Applications. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS), Lausanne, Switzerland, 22–24 October 2014.
- Jones, K.E.; Campbell, P.K.; Normann, R.A. A glass/silicon composite intracortical electrode array. Ann. Biomed. Eng. 1992, 20, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bhandari, R.; Klein, M.; Negi, S.; Rieth, L.; Tathireddy, P.; Toepper, M.; Oppermann, H.; Solzbacher, F. Integrated wireless neural interface based on the Utah electrode array. Biomed. Microdevices 2009, 11, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.L.; D’Stefan, D.J. Controlled Etching of Silicon in the HF-HN03 System. J. Electrochem. Soc. 1962, 109, 37–42. [Google Scholar] [CrossRef]
- Bhandari, R.; Negi, S.; Rieth, L.; Solzbacher, F. A Wafer-Scale Etching Technique for High Aspect Ratio Implantable MEMS Structures. Sens. Actuators A Phys. 2010, 162, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.M.; Rieth, L.; Normann, R.A.; Tathireddy, P.; Solzbacher, F. Encapsulation of an integrated neural interface device with Parylene C. IEEE Trans. Biomed. Eng. 2009, 56, 23–29. [Google Scholar] [PubMed]
- Brown, B.; Parker, C.B.; Stoner, B.R.; Glass, J.T. Growth of vertically aligned bamboo-like carbon nanotubes from ammonia/methane precursors using a platinum catalyst. Carbon 2011, 49, 266–274. [Google Scholar] [CrossRef]
- Nguyen-Vu, T.D.; Chen, H.; Cassell, A.M.; Andrews, R.; Meyyappan, M.; Li, J. Vertically aligned carbon nanofiber arrays: An advance toward electrical-neural interfaces. Small 2006, 2, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Teo, K.B.K.; Ducati, C.; Rupesinghe, R.L.; Amaratunga, G.A.J.; Ferrari, A.C.; Roy, D.; Robertson, J.; Milne, W.I. Growth process conditions of vertically aligned carbon nanotubes using plasma enhanced chemical vapor deposition. J. Appl. Phys. 2001, 90, 5308–5317. [Google Scholar] [CrossRef]
- Meyyappan, M. A review of plasma enhanced chemical vapour deposition of carbon nanotubes. J. Phys. D Appl. Phys. 2009, 42, 21. [Google Scholar] [CrossRef]
- Rose, T.L.; Robblee, L.S. Electrical stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses. IEEE Trans. Biomed. Eng. 1990, 37, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Hudak, E.M.; Mortimer, J.T.; Martin, H.B. Platinum for neural stimulation: Voltammetry considerations. J. Neural Eng. 2010, 7, 26005. [Google Scholar] [CrossRef] [PubMed]
- Slavcheva, E.; Vitushinsky, R.; Mokwa, W.; Schenakenberg, U. Sputtered iridium oxide films as charge injection material for functional electrostimulation. J. Electrochem. Soc. 2004, 151, E226–E237. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raut, R.; Sawan, M. Energy-Efficient High-Voltage Compliant Implantable Brain-Machine Interfaces. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS), Rotterdam, The Netherlands, 31 October–2 November 2013; pp. 81–84.
- Hasanuzzaman, M.; Raut, R.; Sawan, M. High-voltage compliant microelectrode array drivers for intracortical microstimulation. Int. J. Circuit Theory Appl. 2016, 44, 660–682. [Google Scholar] [CrossRef]
- Robinson, D.A. The electrical properties of metal microelectrodes. Proc. IEEE 1968, 56, 1065–1071. [Google Scholar] [CrossRef]
- Sawan, M.; Laaziri, Y.; Mounaim, F.; Elzayat, E.; Corcos, J.; Elhilali, M.M. Electrode-tissues interface: Modeling and experimental validation. Biomed. Mater. 2007, 2, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley: Malden, MA, USA, 2005. [Google Scholar]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 15007. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Negi, S.; Davis, T.; Keefer, E.D.; Wiggins, H.; House, P.A.; Greger, B. The Use of a Novel Carbon Nanotube Coated Microelectrode Array for Chronic Intracortical Recording and Microstimulation. In Proceedings of the IEEE Annual International Conference on Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012.
- Bhandari, R.; Negi, S.; Rieth, L.; Normann, R.A.; Solzbacher, F. A novel method of fabricating convoluted shaped electrode arrays for neural and retinal prostheses. Sens. Actuators A Phys. 2008, 145, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Abidian, M.R.; Martin, D.C. Multifunctional Nanobiomaterials for Neural Interfaces. Adv. Funct. Mater. 2009, 19, 573–585. [Google Scholar] [CrossRef]
- Weiland, J.D.; Anderson, D.J.; Humayun, M.S. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Trans. Biomed. Eng. 2002, 49, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Shimada, A.; Torimitsu, K. Ion conducting polymer microelectrodes for interfacing with neural networks. J. Neurosci. Methods 2007, 160, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ghane-Motlagh, B.; Javanbakht, T.; Shoghi, F.; Wilkinson, K.J.; Martel, R.; Sawan, M. Physicochemical properties of peptide-conjugated microelectrode arrays and their in vitro effects on Neuroblast cells. J. Mater. Sci. Eng. C 2016, 68, 642–650. [Google Scholar] [CrossRef] [PubMed]













| Metal | Ambient | Chamber Pressure (mTorr) | Power (W) | Time (min) | Thickness (nm) |
|---|---|---|---|---|---|
| Ti | Ar | 10 | 90 | 11 | 100 ± 5 |
| Pt | Ar | 10 | 90 | 16 | 400 ± 10 |
| Conventional Method [32] | This Work |
|---|---|
First Masking Process:
| Single masking process:
|
Second Masking Process:
|
| Coating | α | A (F) | RCT (kΩ) | Cs (F) |
|---|---|---|---|---|
| Pt | 0.85 | 2.30 × 10−8 | 1900 | 9 × 10−11 |
| CNTs | 0.77 | 7.20 × 10−6 | 24 | 8.60 × 10−9 |
| MEAs | Geometry | Characteristics | ||||
|---|---|---|---|---|---|---|
| Tip-Coating | Z (kΩ) at 1 kHz | Qinj (mC/cm2) | QCDC (mC/cm2) | Density (Electrodes/1.96 mm2) | ||
| [46,47] | 2D Quasi-3D | Pt, SIROF 1, CNTs 2 | Pt: 125 SIROF: 6 CNTs: 49.7 Electrode tip surface area: 2 × 10−5 cm2 | Pt: 0.3 SIROF: 2 CNTs 3 | Pt: 4.4 ± 3.1 SIRO: 34.3 ± 21.7 CNTs: 1217.359 nC | 16 |
| [22] | NA | Pt/W 4 microwire | CVD-CNT: 15 PPy-CNT 5: 7 Au-CNT: 50 Electrode tip surface area: 6.5 × 10−5 cm2 | CVD-CNT: 4 PPy-CNT: 7 Au-CNT: 0.8 | CVD-CNT: 1743.4 ± 161.7 PPy-CNT: 2920.3 ± 191.4 | Single probe |
| This work | 3D | Pt, CNTs 6 | Pt: 70 CVD-CNTs: 14 Electrode tip surface area: 1.6 × 10−5 cm2 | Pt: 0.3 CNTs: 10 | Pt: 21.7 ± 10.2 CNTs: 13020 ± 6510.2 | 25 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghane Motlagh, B.; Choueib, M.; Hajhosseini Mesgar, A.; Hasanuzzaman, M.; Sawan, M. Direct Growth of Carbon Nanotubes on New High-Density 3D Pyramid-Shaped Microelectrode Arrays for Brain-Machine Interfaces. Micromachines 2016, 7, 163. https://doi.org/10.3390/mi7090163
Ghane Motlagh B, Choueib M, Hajhosseini Mesgar A, Hasanuzzaman M, Sawan M. Direct Growth of Carbon Nanotubes on New High-Density 3D Pyramid-Shaped Microelectrode Arrays for Brain-Machine Interfaces. Micromachines. 2016; 7(9):163. https://doi.org/10.3390/mi7090163
Chicago/Turabian StyleGhane Motlagh, Bahareh, May Choueib, Alireza Hajhosseini Mesgar, Md. Hasanuzzaman, and Mohamad Sawan. 2016. "Direct Growth of Carbon Nanotubes on New High-Density 3D Pyramid-Shaped Microelectrode Arrays for Brain-Machine Interfaces" Micromachines 7, no. 9: 163. https://doi.org/10.3390/mi7090163