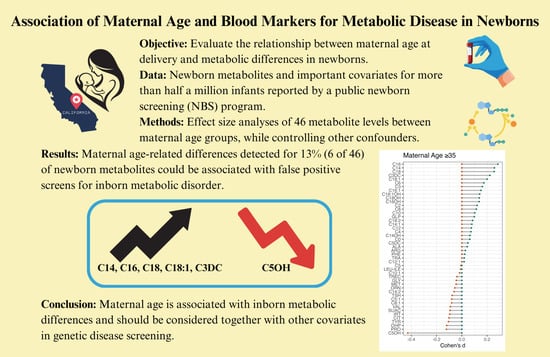
Association of Maternal Age and Blood Markers for Metabolic Disease in Newborns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Summary and Preprocessing
2.2. Analysis of Maternal Age
2.3. Analysis of Maternal Age in Relation to Other Variables
2.4. Analysis of Maternal Age-Related Differences and False-Positive Results
2.5. Statistical Analyses and Software
3. Results
3.1. Identification of Metabolic Differences Related to Maternal Age
3.2. Correlation of Maternal Age-Related Differences to False-Positive Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathews, T.J.; Hamilton, B.E. Mean Age of Mother, 1970–2000. Natl. Vital Stat. Rep. 2002, 51, 1–13. [Google Scholar] [PubMed]
- Osterman, M.; Hamilton, B.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2020. Natl. Vital Stat. Rep. 2021, 70, 17. [Google Scholar]
- Matthews, T.J.; Hamilton, B.E. First Births to Older Women Continue to Rise. NCHS Data Brief 2014, 152, 1–8. [Google Scholar]
- Laopaiboon, M.; Lumbiganon, P.; Intarut, N.; Mori, R.; Ganchimeg, T.; Vogel, J.P.; Souza, J.P.; Gülmezoglu, A.M. WHO Multicountry Survey on Maternal Newborn Health Research Network Advanced Maternal Age and Pregnancy Outcomes: A Multicountry Assessment. BJOG 2014, 121 (Suppl. 1), 49–56. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.F.; Bradshaw, L.; Bugg, G.J.; Thornton, J.G. Causes of Antepartum Stillbirth in Women of Advanced Maternal Age. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Claramonte Nieto, M.; Meler Barrabes, E.; Garcia Martínez, S.; Gutiérrez Prat, M.; Serra Zantop, B. Impact of Aging on Obstetric Outcomes: Defining Advanced Maternal Age in Barcelona. BMC Pregnancy Childbirth 2019, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Pregnancy at Age 35 Years or Older: ACOG Obstetric Care Consensus No. 11. Obstet. Gynecol. 2022, 140, 348–366. [Google Scholar]
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int. J. Womens Health 2021, 13, 751–759. [Google Scholar] [CrossRef]
- Egan, J.F.X.; Smith, K.; Timms, D.; Bolnick, J.M.; Campbell, W.A.; Benn, P.A. Demographic Differences in Down Syndrome Livebirths in the US from 1989 to 2006. Prenat. Diagn. 2011, 31, 389–394. [Google Scholar] [CrossRef]
- Who Is at Risk for Down Syndrome? Available online: https://www.nichd.nih.gov/health/topics/down/conditioninfo/Risks (accessed on 20 July 2023).
- Frederiksen, L.E.; Ernst, A.; Brix, N.; Braskhøj Lauridsen, L.L.; Roos, L.; Ramlau-Hansen, C.H.; Ekelund, C.K. Risk of Adverse Pregnancy Outcomes at Advanced Maternal Age. Obstet. Gynecol. 2018, 131, 457–463. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Shchelochkov, O.A.; Cook, D.E.; Berberich, S.L.; Copeland, S.; Dagle, J.M.; Murray, J.C. The Influence of Maternal Disease on Metabolites Measured as Part of Newborn Screening. J. Matern. Fetal. Neonatal Med. 2013, 26, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.-P.; Rezzi, S.; Lussu, M.; Pintus, R.; Pattumelli, M.G.; Noto, A.; Dessì, A.; Da Silva, L.; Collino, S.; Ciccarelli, S.; et al. Urinary Metabolomics in Term Newborns Delivered Spontaneously or with Cesarean Section: Preliminary Data. J. Pediatr. Neonatal Indivi 2018, 7, e070219. [Google Scholar]
- Perrone, S.; Laschi, E.; De Bernardo, G.; Giordano, M.; Vanacore, F.; Tassini, M.; Calderisi, M.; Toni, A.L.; Buonocore, G.; Longini, M. Newborn Metabolomic Profile Mirrors That of Mother in Pregnancy. Med. Hypotheses 2020, 137, 109543. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, Y.; Oropeza-Valdez, J.J.; Blanco-Sandate, J.O.; Herrera-Van Oostdam, A.S.; Zheng, J.; Chi Guo, A.; Lima-Rogel, V.; Rajabzadeh, R.; Salgado-Bustamante, M.; Adrian-Lopez, J.; et al. The Urinary Metabolome of Healthy Newborns. Metabolites 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.T.; Ryckman, K.K.; Baer, R.J.; Charlton, M.E.; Breheny, P.J.; Terry, W.W.; Kober, K.; Oltman, S.; Rogers, E.E.; Jelliffe-Pawlowski, L.L.; et al. Metabolic Differences among Newborns Born to Mothers with a History of Leukemia or Lymphoma. J. Matern. Fetal. Neonatal Med. 2022, 35, 6751–6758. [Google Scholar] [CrossRef] [PubMed]
- Cajachagua-Torres, K.N.; Blaauwendraad, S.M.; El Marroun, H.; Demmelmair, H.; Koletzko, B.; Gaillard, R.; Jaddoe, V.W.V. Fetal Exposure to Maternal Smoking and Neonatal Metabolite Profiles. Metabolites 2022, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Cicalini, I.; Moffa, S.; Tommolini, M.L.; Valentinuzzi, S.; Zucchelli, M.; Bucci, I.; Chiacchiaretta, P.; Fontana, A.; Federici, L.; De Laurenzi, V.; et al. Impact of Maternal Lifestyle and Dietary Habits during Pregnancy on Newborn Metabolic Profile. Nutrients 2023, 15, 2297. [Google Scholar] [CrossRef] [PubMed]
- American College of Medical Genetics Newborn Screening Expert Group Newborn Screening: Toward a Uniform Screening Panel and System—Executive Summary. Pediatrics 2006, 117, S296–S307. [CrossRef]
- Newborn Screening. Available online: https://www.nichd.nih.gov/health/topics/factsheets/newborn (accessed on 20 July 2023).
- Rose, N.C.; Dolan, S.M. Newborn Screening and the Obstetrician. Obstet. Gynecol. 2012, 120, 908–917. [Google Scholar] [CrossRef]
- McHugh, D.M.S.; Cameron, C.A.; Abdenur, J.E.; Abdulrahman, M.; Adair, O.; Al Nuaimi, S.A.; Åhlman, H.; Allen, J.J.; Antonozzi, I.; Archer, S.; et al. Clinical Validation of Cutoff Target Ranges in Newborn Screening of Metabolic Disorders by Tandem Mass Spectrometry: A Worldwide Collaborative Project. Genet. Med. 2011, 13, 230–254. [Google Scholar] [CrossRef]
- Lachenbruch, P.A.; Cohen, J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). J. Am. Stat. Assoc. 1989, 84, 1096. [Google Scholar] [CrossRef]
- Peng, G.; Tang, Y.; Cowan, T.M.; Zhao, H.; Scharfe, C. Timing of Newborn Blood Collection Alters Metabolic Disease Screening Performance. Front. Pediatr. 2020, 8, 623184. [Google Scholar] [CrossRef] [PubMed]
- Hook, E.B. Rates of Chromosome Abnormalities at Different Maternal Ages. Obstet. Gynecol. 1981, 58, 282–285. [Google Scholar] [PubMed]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. Available online: https://dplyr.tidyverse.org (accessed on 6 June 2023).
- Maintainer, M. Package “Effsize”. Available online: https://cran.r-project.org/web/packages/effsize/effsize.pdf (accessed on 20 July 2023).
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.A.M.; Chen, Z.J. Ggplot2: Elegant Graphics for Data Analysis (2nd ed.). Measurement 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Kassambara, A. “ggplot2” Based Publication Ready Plots. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 6 June 2023).
- Wood, S.N.; Pya, N.; Säfken, B. Smoothing Parameter and Model Selection for General Smooth Models. J. Am. Stat. Assoc. 2016, 111, 1548–1563. [Google Scholar] [CrossRef]
- Analysis of Variance—ANOVA. Statistics and Data Analysis for Microarrays Using R and Bioconductor; Chapman and Hall/CRC: Boca Raton, FL, USA, 2016; pp. 441–478. ISBN 9780429130588. [Google Scholar]
- Massey, F.J., Jr. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Newcombe, R.G. Interval Estimation for the Difference between Independent Proportions: Comparison of Eleven Methods. Stat. Med. 1998, 17, 873–890. [Google Scholar] [CrossRef]
- Blanco, C.L.; Gong, A.K.; Green, B.K.; Falck, A.; Schoolfield, J.; Liechty, E.A. Early Changes in Plasma Amino Acid Concentrations during Aggressive Nutritional Therapy in Extremely Low Birth Weight Infants. J. Pediatr. 2011, 158, 543–548.e1. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Banks, K.; Gaviglio, A.; Hietala, A.; McCann, M.; Thomas, W. Comparison of One-Tier and Two-Tier Newborn Screening Metrics for Congenital Adrenal Hyperplasia. Pediatrics 2012, 130, e1261–e1268. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Berberich, S.L.; Shchelochkov, O.A.; Cook, D.E.; Murray, J.C. Clinical and Environmental Influences on Metabolic Biomarkers Collected for Newborn Screening. Clin. Biochem. 2013, 46, 133–138. [Google Scholar] [CrossRef]
- Hall, P.L.; Marquardt, G.; McHugh, D.M.S.; Currier, R.J.; Tang, H.; Stoway, S.D.; Rinaldo, P. Postanalytical Tools Improve Performance of Newborn Screening by Tandem Mass Spectrometry. Genet. Med. 2014, 16, 889–895. [Google Scholar] [CrossRef]
- Clark, R.H.; Kelleher, A.S.; Chace, D.H.; Spitzer, A.R. Gestational Age and Age at Sampling Influence Metabolic Profiles in Premature Infants. Pediatrics 2014, 134, e37–e46. [Google Scholar] [CrossRef]
- Peng, G.; de Fontnouvelle, C.A.; Enns, G.M.; Cowan, T.M.; Zhao, H.; Scharfe, C. Elevated Methylmalonic Acidemia (MMA) Screening Markers in Hispanic and Preterm Newborns. Mol. Genet. Metab. 2019, 126, 39–42. [Google Scholar] [CrossRef]
- Peng, G.; Tang, Y.; Gandotra, N.; Enns, G.M.; Cowan, T.M.; Zhao, H.; Scharfe, C. Ethnic Variability in Newborn Metabolic Screening Markers Associated with False-Positive Outcomes. J. Inherit. Metab. Dis. 2020, 43, 934–943. [Google Scholar] [CrossRef]
- Ernst, M.; Rogers, S.; Lausten-Thomsen, U.; Björkbom, A.; Laursen, S.S.; Courraud, J.; Børglum, A.; Nordentoft, M.; Werge, T.; Mortensen, P.B.; et al. Gestational Age-Dependent Development of the Neonatal Metabolome. Pediatr. Res. 2021, 89, 1396–1404. [Google Scholar] [CrossRef]
- Mansell, T.; Vlahos, A.; Collier, F.; Ponsonby, A.-L.; Vuillermin, P.; Ellul, S.; Tang, M.L.K.; Burgner, D.; Saffery, R. Barwon Infant Study Investigator team The Newborn Metabolome: Associations with Gestational Diabetes, Sex, Gestation, Birth Mode, and Birth Weight. Pediatr. Res. 2022, 91, 1864–1873. [Google Scholar] [CrossRef]
- Baumgartner, C.; Böhm, C.; Baumgartner, D.; Marini, G.; Weinberger, K.; Olgemöller, B.; Liebl, B.; Roscher, A.A. Supervised Machine Learning Techniques for the Classification of Metabolic Disorders in Newborns. Bioinformatics 2004, 20, 2985–2996. [Google Scholar] [CrossRef]
- Van den Bulcke, T.; Vanden Broucke, P.; Van Hoof, V.; Wouters, K.; Vanden Broucke, S.; Smits, G.; Smits, E.; Proesmans, S.; Van Genechten, T.; Eyskens, F. Data Mining Methods for Classification of Medium-Chain Acyl-CoA Dehydrogenase Deficiency (MCADD) Using Non-Derivatized Tandem MS Neonatal Screening Data. J. Biomed. Inform. 2011, 44, 319–325. [Google Scholar] [CrossRef]
- Marquardt, G.; Currier, R.; McHugh, D.M.S.; Gavrilov, D.; Magera, M.J.; Matern, D.; Oglesbee, D.; Raymond, K.; Rinaldo, P.; Smith, E.H.; et al. Enhanced Interpretation of Newborn Screening Results without Analyte Cutoff Values. Genet. Med. 2012, 14, 648–655. [Google Scholar] [CrossRef]
- Tortorelli, S.; Eckerman, J.S.; Orsini, J.J.; Stevens, C.; Hart, J.; Hall, P.L.; Alexander, J.J.; Gavrilov, D.; Oglesbee, D.; Raymond, K.; et al. Moonlighting Newborn Screening Markers: The Incidental Discovery of a Second-Tier Test for Pompe Disease. Genet. Med. 2018, 20, 840–846. [Google Scholar] [CrossRef]
- Minter Baerg, M.M.; Stoway, S.D.; Hart, J.; Mott, L.; Peck, D.S.; Nett, S.L.; Eckerman, J.S.; Lacey, J.M.; Turgeon, C.T.; Gavrilov, D.; et al. Precision Newborn Screening for Lysosomal Disorders. Genet. Med. 2018, 20, 847–854. [Google Scholar] [CrossRef]
- Peng, G.; Tang, Y.; Cowan, T.M.; Enns, G.M.; Zhao, H.; Scharfe, C. Reducing False-Positive Results in Newborn Screening Using Machine Learning. Int. J. Neonatal Screen. 2020, 6, 16. [Google Scholar] [CrossRef]
- Zaunseder, E.; Haupt, S.; Mütze, U.; Garbade, S.F.; Kölker, S.; Heuveline, V. Opportunities and Challenges in Machine Learning-Based Newborn Screening-A Systematic Literature Review. JIMD Rep. 2022, 63, 250–261. [Google Scholar] [CrossRef]
- Peng, G.; Pakstis, A.J.; Gandotra, N.; Cowan, T.M.; Zhao, H.; Kidd, K.K.; Scharfe, C. Metabolic Diversity in Human Populations and Correlation with Genetic and Ancestral Geographic Distances. Mol. Genet. Metab. 2022, 137, 292–300. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef]
- McMahon, R.; DeMartino, L.; Sowizral, M.; Powers, D.; Tracy, M.; Caggana, M.; Tavakoli, N.P. The Impact of Seasonal Changes on Thyroxine and Thyroid-Stimulating Hormone in Newborns. Int. J. Neonatal Screen. 2021, 7, 8. [Google Scholar] [CrossRef]
- Henderson, M.P.A.; McIntosh, N.; Chambers, A.; Desormeaux, E.; Kowalski, M.; Milburn, J.; Chakraborty, P. Biotinidase Activity Is Affected by Both Seasonal Temperature and Filter Collection Cards. Clin. Biochem. 2023, 115, 129–136. [Google Scholar] [CrossRef]
- Soneji, S.; Beltrán-Sánchez, H. Association of Maternal Cigarette Smoking and Smoking Cessation with Preterm Birth. JAMA Netw. Open 2019, 2, e192514. [Google Scholar] [CrossRef]
- Tatsuta, N.; Asato, K.; Anai, A.; Suzuki, T.; Sakurai, K.; Ota, C.; Arima, T.; Sugawara, J.; Yaegashi, N.; Nakai, K.; et al. Timing of Maternal Smoking Cessation and Newborn Weight, Height, and Head Circumference. Obstet. Gynecol. 2023, 141, 119–125. [Google Scholar] [CrossRef]
- Porta, F.; Mussa, A.; Ponzone, A. Breastfeeding Effects on Newborn Screening. J. Pediatr. 2010, 156, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Bass, H.N.; Taylor, J.B. Perinatal Screening for Congenital Malformations and Genetic Disorders: Current Status and Future Directions. Perm. J. 2002, 6, 15–20. [Google Scholar] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Peng, G.; Zhao, H.; Scharfe, C. Association of Maternal Age and Blood Markers for Metabolic Disease in Newborns. Metabolites 2024, 14, 5. https://doi.org/10.3390/metabo14010005
Xie Y, Peng G, Zhao H, Scharfe C. Association of Maternal Age and Blood Markers for Metabolic Disease in Newborns. Metabolites. 2024; 14(1):5. https://doi.org/10.3390/metabo14010005
Chicago/Turabian StyleXie, Yuhan, Gang Peng, Hongyu Zhao, and Curt Scharfe. 2024. "Association of Maternal Age and Blood Markers for Metabolic Disease in Newborns" Metabolites 14, no. 1: 5. https://doi.org/10.3390/metabo14010005