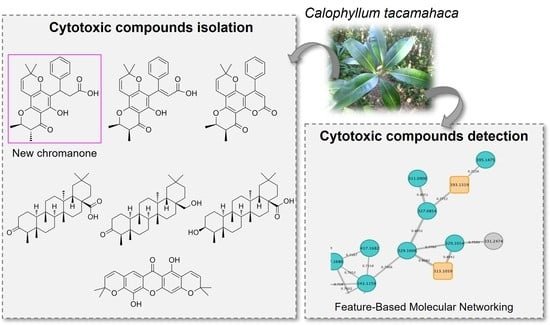
Cytotoxic Metabolites from Calophyllum tacamahaca Willd.: Isolation and Detection through Feature-Based Molecular Networking
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Molecular Modeling
2.4.1. Calculation of Averaged NMR Spectra
2.4.2. Conformational Study for UV–ECD Calculations
2.4.3. Calculation of Averaged UV and ECD Spectra
2.5. In Vitro Cytotoxic Assay
2.6. Feature-Based Molecular Networking
3. Results and Discussion
3.1. Isolation of Compounds 1–14
3.2. Structure Elucidation of Isocaloteysmannic Acid (1)
3.3. Absolute Configuration of Isocaloteysmannic Acid (1)
3.4. Cytotoxic Activity of the Isolated Compounds
3.5. Feature-Based Molecular Networking Analysis of the Crude Extract
3.5.1. Chemodiversity of the Species
3.5.2. Detection of Additional Bioactive Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Gupta, S.; Gupta, P. The Genus Calophyllum: Review of Ethnomedicinal Uses, Phytochemistry and Pharmacology. In Bioactive Natural Products in Drug Discovery; Singh, J., Meshram, V., Gupta, M., Eds.; Springer: Singapore, 2020; pp. 215–242. ISBN 9789811513930. [Google Scholar]
- Zamakshshari, N.H.; Ee, G.C.L.; Ismail, I.S.; Ibrahim, Z.; Mah, S.H. Cytotoxic Xanthones Isolated from Calophyllum Depressinervosum and Calophyllum Buxifolium with Antioxidant and Cytotoxic Activities. Food Chem. Toxicol. 2019, 133, 110800. [Google Scholar] [CrossRef] [PubMed]
- Leu, T.; Raharivelomanana, P.; Soulet, S.; Bianchini, J.P.; Herbette, G.; Faure, R. New Tricyclic and Tetracyclic Pyranocoumarins with an Unprecedented C-4 Substituent. Structure Elucidation of Tamanolide, Tamanolide D and Tamanolide P from Calophyllum inophyllum of French Polynesia. Magn. Reson. Chem. 2009, 47, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Zailan, A.A.D.; Karunakaran, T.; Bakar, M.H.A.; Mian, V.J.Y. The Malaysian Genus Calophyllum (Calophyllaceae): A Review on Its Phytochemistry and Pharmacological Activities. Nat. Prod. Res. 2022; in press. [Google Scholar] [CrossRef]
- Leu, T. Contribution à la Connaissance de la Flore Polynésienne: ÉVALUATION de L’intérêt Pharmacologique de Quelques Plantes Médicinales et Étude Phytochimique du Tamanu (Calophyllum inophyllum, L.—Clusiaceae). Ph.D. Thesis, Université de la Polynésie Française, Tahiti, French Polynesia, 2009. [Google Scholar]
- Gómez-Verjan, J.C.; Rodríguez-Hernández, K.D.; Reyes-Chilpa, R. Bioactive Coumarins and Xanthones From Calophyllum Genus and Analysis of Their Druglikeness and Toxicological Properties. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 53, pp. 277–307. ISBN 978-0-444-63930-1. [Google Scholar]
- Dorla, E.; Grondin, I.; Hue, T.; Clerc, P.; Dumas, S.; Gauvin-Bialecki, A.; Laurent, P. Traditional Uses, Antimicrobial and Acaricidal Activities of 20 Plants Selected among Reunion Island’s Flora. S. Afr. J. Bot. 2019, 122, 447–456. [Google Scholar] [CrossRef]
- Adsersen, A.; Adsersen, H. Plants from Réunion Island with Alleged Antihypertensive and Diuretic Effects—An Experimental and Ethnobotanical Evaluation. J. Ethnopharmacol. 1997, 58, 189–206. [Google Scholar] [CrossRef]
- Ledoux, A.; Cao, M.; Jansen, O.; Mamede, L.; Campos, P.-E.; Payet, B.; Clerc, P.; Grondin, I.; Girard-Valenciennes, E.; Hermann, T.; et al. Antiplasmodial, Anti-Chikungunya Virus and Antioxidant Activities of 64 Endemic Plants from the Mascarene Islands. Int. J. Antimicrob. Agents 2018, 52, 622–628. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.C.; Covington, C.D.; Fuller, R.W.; Bokesch, H.R.; Young, S.; Cardellina, J.H.; Kadushin, M.R.; Soejarto, D.D.; Stevens, P.F.; Cragg, G.M.; et al. Pyranocoumarins from Tropical Species of the Genus Calophyllum: A Chemotaxonomic Study of Extracts in the National Cancer Institute Collection. J. Nat. Prod. 1998, 61, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, A.E. Evaluation of Antioxidant and Anti-Inflammatory Effects of Medicinal Plants from Reunion Island against Obesity-Related Disorders; Université de Liège: Liège, Belgium, 2014; p. 46. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2013; Available online: http://www.gaussian.com (accessed on 1 June 2022).
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- AMPAC 11, 1992–2017 Semichem, Inc. 12456 W 62nd Terrace—Suite D, Shawnee, KS, USA, 66216. Available online: http://www.semichem.com (accessed on 6 June 2022).
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Olivon, F.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MZmine 2 Data-Preprocessing To Enhance Molecular Networking Reliability. Anal. Chem. 2017, 89, 7836–7840. [Google Scholar] [CrossRef]
- Smirnov, A.; Jia, W.; Walker, D.I.; Jones, D.P.; Du, X. ADAP-GC 3.2: Graphical Software Tool for Efficient Spectral Deconvolution of Gas Chromatography–High-Resolution Mass Spectrometry Metabolomics Data. J. Proteome Res. 2018, 17, 470–478. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Allard, P.-M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.-L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Yang, N.-Y.; Tao, W.-W.; Duan, J.-A. Antithrombotic Flavonoids from the Faeces of Trogopterus xanthipes. Nat. Prod. Res. 2010, 24, 1843–1849. [Google Scholar] [CrossRef]
- Kijjoa, A.; Gonzalez, M.J.; Afonso, C.M.; Pinto, M.M.M.; Anantachoke, C.; Herz, W. Xanthones from Calophyllum teysmannii Var. Inophylloide. Phytochemistry 2000, 53, 1021–1024. [Google Scholar] [CrossRef]
- Ito, C.; Miyamoto, Y.; Rao, K.S.; Furukawa, H. A Novel Dibenzofuran and Two New Xanthones from Calophyllum Panciflorum. Chem. Pharm. Bull. 1996, 44, 441–443. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R. The Inophyllums, Novel Inhibitors of HIV-1 Reverse Transcriptase Isolated from the Malaysian Tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Kawazu, K.; Ohigashi, H.; Mitsui, T. The Psiscicidal Constituents of Calophyllum inophyllum Linn. Tetrahedron Lett. 1968, 19, 2383–2385. [Google Scholar] [CrossRef]
- Laure, F.; Herbette, G.; Faure, R.; Bianchini, J.P.; Raharivelomanana, P.; Fogliani, B. Structures of New Secofriedelane and Friedelane Acids from Calophyllum inophyllum of French Polynesia. Magn. Reson. Chem. 2005, 43, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-G.; Lim, T.-B.; Sim, K.-Y.; Goh, S.H. A Highly Prenylated Xanthone from the Bark of Calophyllum gracilipes (Guttiferae). Nat. Prod. Lett. 1997, 10, 55–58. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Ebajo, V., Jr.; Brkljača, R.; Urban, S. Triterpenes from Calophyllum inophyllum Linn. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 718–722. [Google Scholar]
- Li, X.J.; Liu, Z.Z.; Kim, K.-W.; Wang, X.; Li, Z.; Kim, Y.-C.; Yook, C.S.; Liu, X.Q. Chemical Constituents from Leaves of Pileostegia viburnoides Hook.f.et Thoms. Nat. Prod. Sci. 2016, 22, 154–161. [Google Scholar] [CrossRef]
- Jackson, B.; Locksley, H.D.; Scheinwans, F. Extractives from Guttiferae—VIII. The Isolation of 6-(3,3-Dimethylallyl)-1,5-Dihydroxyxanthone from Calophyllum Scriblitifolium Henderson and Wyatt-Smith. Tetrahedron 1967, 24, 3059–3068. [Google Scholar] [CrossRef]
- Lim, C.K.; Subramaniam, H.; Say, Y.H.; Jong, V.Y.M.; Khaledi, H.; Chee, C.F. A New Chromanone Acid from the Stem Bark of Calophyllum teysmannii. Nat. Prod. Res. 2015, 29, 1970–1977. [Google Scholar] [CrossRef]
- Huitric, A.C.; Carr, J.B.; Trager, W.F.; Nist, B.J. Configurational and Conformational Analysis. Tetrahedron 1963, 19, 2145–2151. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds Using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L.; De Silva, A.M.Y.J.; Sotheeswaran, S.; Balasubramaniam, S.; Wazeer, M.I.M. Terpenoid and Biflavonoid Constituents of Calophyllum Calaba and Garcinia Spicata from Sri Lanka. Phytochemistry 1984, 23, 323–328. [Google Scholar] [CrossRef]







| Position | δH m (J in Hz) | δC |
|---|---|---|
| 2 | - | 177.2 |
| 3 | 3.07, dd (15.2, 7.2) 3.27, dd (15.2, 8.2) | 38.2 |
| 4 | 5.0, 7 brt a (7.7) | 36.3 |
| 4a | - | 113.0 |
| 4b | - | 160.9 |
| 6 | - | 79.2 |
| 7 | 5.48, d (10.0) | 127.3 |
| 8 | 6.49, d (10.0) | 116.7 |
| 8a | - | 102.9 |
| 8b | - | 156.8 |
| 10 | 4.18, dq (11.3, 6.2) | 80.3 |
| 11 | 2.61, dq (11.3, 6.9) | 46.9 |
| 12 | - | 200.7 |
| 12a | - | 102.6 |
| 12b | - | 162.2 |
| 13 | 1.01, s | 27.5 |
| 14 | 1.41, s | 28.5 |
| 15 | 1.49, d (6.2) | 19.8 |
| 16 | 1.19, d (6.9) | 10.3 |
| 1′ | - | 145.2 |
| 2′, 6′ | 7.33, d (7.6) | 128.8 |
| 3′, 5′ | 7.20, brt (7.5) | 128.8 |
| 4′ | 7.10, brt (7.3) | 126.7 |
| Compound | IC50 (µg/mL) a | |
|---|---|---|
| HepG2 | HT29 | |
| 1 | 19.65 ± 2.34 | 25.68 ± 2.08 |
| 2 | 39.03 ± 3.23 | 41.97 ± 2.54 |
| 6 | 15.98 ± 3.65 | 18.97 ± 2.94 |
| 7 | 2.44 ± 0.67 | 4.24 ± 0.67 |
| 8 | 7.03 ± 1.56 | 5.94 ± 0.07 |
| 9 | 45.09 ± 2.09 | 56.98 ± 3.76 |
| 10 | 9.54 ± 1.22 | 10.46 ± 2.08 |
| 11 | 3.34 ± 0.94 | 5.97 ± 0.99 |
| 12 | 15.38 ± 2.07 | 10.26 ± 1.34 |
| 13 | 6.65 ± 1.54 | 4.06 ± 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerometta, E.; Herbette, G.; Garayev, E.; Marvilliers, A.; Naubron, J.-V.; Di Giorgio, C.; Campos, P.-E.; Clerc, P.; Ledoux, A.; Frederich, M.; et al. Cytotoxic Metabolites from Calophyllum tacamahaca Willd.: Isolation and Detection through Feature-Based Molecular Networking. Metabolites 2023, 13, 582. https://doi.org/10.3390/metabo13050582
Gerometta E, Herbette G, Garayev E, Marvilliers A, Naubron J-V, Di Giorgio C, Campos P-E, Clerc P, Ledoux A, Frederich M, et al. Cytotoxic Metabolites from Calophyllum tacamahaca Willd.: Isolation and Detection through Feature-Based Molecular Networking. Metabolites. 2023; 13(5):582. https://doi.org/10.3390/metabo13050582
Chicago/Turabian StyleGerometta, Elise, Gaëtan Herbette, Elnur Garayev, Arnaud Marvilliers, Jean-Valère Naubron, Carole Di Giorgio, Pierre-Eric Campos, Patricia Clerc, Allison Ledoux, Michel Frederich, and et al. 2023. "Cytotoxic Metabolites from Calophyllum tacamahaca Willd.: Isolation and Detection through Feature-Based Molecular Networking" Metabolites 13, no. 5: 582. https://doi.org/10.3390/metabo13050582