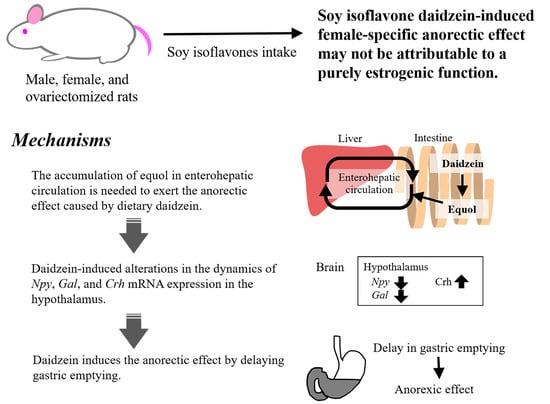
Mechanism of Soy Isoflavone Daidzein-Induced Female-Specific Anorectic Effect
Abstract
:1. Introduction
2. The Anorectic Effect May Not Be Caused by Estrogenic Properties of Soy Isoflavones
3. Certain Amount of Equol Accumulation in Enterohepatic Circulation May Be Required to Exhibit the Anorectic Effect Caused by Dietary Daidzein
4. Daidzein Alters Gene Expression of Hypothalamic Appetite-Related Neuropeptides
5. Daidzein Induces the Anorectic Effect by Delaying Gastric Emptying
6. Conclusions and Future Prospectus
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and Western Diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999, 70, 439s–450s. [Google Scholar] [CrossRef] [Green Version]
- Kurzer, M.S.; Xu, X. Dietary Phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S. The chemopreventive properties of soy isoflavonoids in animal models of breast cancer. Breast Cancer Res. Treat. 1997, 46, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J.; Loprinzi, C.L. Soy for Breast Cancer Survivors: A Critical Review of the Literature. J. Nutr. 2001, 131 (Suppl. S11), 3095S–3108S. [Google Scholar] [CrossRef] [PubMed]
- Santell, R.C.; Kieu, N.; Helferich, W.G. Genistein inhibits growth of estrogen-independent human breast cancer cells in culture but not in athymic mice. J. Nutr. 2000, 130, 1665–1669. [Google Scholar] [CrossRef] [Green Version]
- Potter, S.M.; Baum, J.A.; Teng, H.; Stillman, R.J.; Shay, N.F.; Erdman, J.W., Jr. Soy protein and isoflavones: Their effects on blood lipids and bone density in postmenopausal women. Am. J. Clin. Nutr. 1998, 68 (Suppl. S6), 1375S–1379S. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, T.; Toda, T.; Tsuji, K.; Ishida, H. Comparative study on reduction of bone loss and lipid metabolism abnormality in ovariectomized rats by soy isoflavones, daidzin, genistin, and glycitin. Biol. Pharm. Bull. 2001, 24, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Picherit, C.; Dalle, M.; Néliat, G.; Lebecque, P.; Davicco, M.J.; Barlet, J.P.; Coxam, V. Genistein and daidzein modulate in vitro rat uterine contractile activity. J. Steroid Biochem. Mol. Biol. 2000, 75, 201–208. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Gutendorf, B.; Westendorf, J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology 2001, 166, 79–89. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of Pure Isoflavones in Healthy Humans and Analysis of Commercial Soy Isoflavone Supplements. J. Nutr. 2001, 131 (Suppl. S4), 1362S–1375S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkelä, S.I.; Pylkkänen, L.H.; Santti, R.S.S.; Adlercreutz, H. Dietary soybean may be antiestrogenic in male mice. J. Nutr. 1995, 125, 437–445. [Google Scholar] [PubMed]
- Lyons, P.M.; Truswell, A.S.; Mira, M.; Vizzard, J.; Abraham, S.F. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am. J. Clin. Nutr. 1989, 49, 1164–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, E.J.; Garrel, D.; Calloway, D.H. Menstrual cycle and voluntary food intake. Am. J. Clin. Nutr. 1989, 49, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Parker, G.C.; McKee, M.E.; Bishop, C.; Coscina, D.V. Whole-body metabolism varies across the estrous cycle in Sprague–Dawley rats. Physiol. Behav. 2001, 74, 399–403. [Google Scholar] [CrossRef]
- Geary, N. Estradiol, CCK and satiation. Peptides 2001, 22, 1251–1263. [Google Scholar] [CrossRef]
- Wade, G.N. Some effects of ovarian hormones on food intake and body weight in female rats. J. Comp. Physiol. Psychol. 1975, 88, 183–193. [Google Scholar] [CrossRef]
- Geary, N.; Asarian, L. Cyclic Estradiol Treatment Normalizes Body Weight and Test Meal Size in Ovariectomized Rats. Physiol. Behav. 1999, 67, 141–147. [Google Scholar] [CrossRef]
- Geary, N.; Asarian, L.; Korach, K.S.; Pfaff, D.W.; Ogawa, S. Deficits in E2-Dependent Control of Feeding, Weight Gain, and Cholecystokinin Satiation in ER-α Null Mice. Endocrinology 2001, 142, 4751–4757. [Google Scholar] [CrossRef]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J. Nutr. 2001, 131, 1202–1206. [Google Scholar] [CrossRef]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake and body composition in postmenopausal women. Menopause 2003, 10, 427–432. [Google Scholar] [CrossRef]
- Kishida, T.; Mizushige, T.; Ohtsu, Y.; Ishikawa, S.; Nagamoto, M.; Izumi, T.; Obata, A.; Ebihara, K. Dietary Soy Isoflavone-Aglycone Lowers Food Intake in Female Rats With and Without Ovariectomy. Obesity 2008, 16, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Herbert, Z.; Kong, J.; Gabrielson, N.; Mautz, A.; Wu, D.; Jirikowski, G.F.; Caldwell, J.D. Estradiol control of expression and levels of estradiol-binding proteins in the medial preoptic area, medial hypothalamus and pituitary. Neuroendocrinology 2003, 78, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cunningham, K.A.; Thomas, M.L. Estrogen regulation of gene expression in the brain: A possible mechanism altering the response to psychostimulants in female rats. Mol. Brain Res. 2002, 100, 75–83. [Google Scholar] [CrossRef]
- De Beun, R.; Jansen, E.; Smeets, M.A.M.; Niesing, J.; Slangen, J.L.; van de Poll, N.E. Estradiol-induced conditioned taste aversion and place aversion in rats: Sex- and dose-dependent effects. Physiol. Behav. 1991, 50, 995–1000. [Google Scholar] [CrossRef]
- Peeters, B.W.M.M.; Smets, R.J.M.; Broekkamp, C.L.E. Sex steroids possess distinct stimulus properties in female and male mice. Brain Res. Bull. 1992, 28, 319–321. [Google Scholar] [CrossRef]
- Bhattarai, K.; Adhikari, S.; Fujitani, M.; Kishida, T. Dietary daidzein, but not genistein, has a hypocholesterolemic effect in non-ovariectomized and ovariectomized female Sprague-Dawley rats on a cholesterol-free diet. Biosci. Biotechnol. Biochem. 2017, 81, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.R.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Bayer, T.; Colnot, T.; Dekant, W. Disposition and biotransformation of the estrogenic isoflavone daidzein in rats. Toxicol. Sci. 2001, 62, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamida, K.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 2006, 102, 247–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr Rev. 2011, 69, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Rachoń, D.; Vortherms, T.; Seidlovä-Wuttke, D.; Wuttke, W. Effects of dietary equol on body weight gain, intra-abdominal fat accumulation, plasma lipids, and glucose tolerance in ovariectomized Sprague-Dawley rats. Menopause 2007, 14, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.; Fabick, K.M.; Setchell, K.D.; Lund, T.D.; Lephart, E.D. Neuromodulation by soy diets or equol: Anti-depressive & anti-obesity-like influences, age- & hormone-dependent effects. BMC Neurosci. 2011, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.M.; Belles, C.A.; Lindley, S.L.; Zimmer-Nechemias, L.D.; Zhao, X.; Witte, D.P.; Kim, M.O.; Setchell, K.D. The chemopreventive action of equol enantiomers in a chemically induced animal model of breast cancer. Carcinogenesis 2010, 31, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Ohtomo, T.; Uehara, M.; Peñalvo, J.L.; Adlercreutz, H.; Katsumata, S.-I.; Suzuki, K.; Takeda, K.; Masuyama, R.; Ishimi, Y. Comparative activities of daidzein metabolites, equol and O-desmethylangolensin, on bone mineral density and lipid metabolism in ovariectomized mice and in osteoclast cell cultures. Eur. J. Nutr. 2008, 47, 273–279. [Google Scholar] [CrossRef]
- Wu, J.; Oka, J.; Ezaki, J.; Ohtomo, T.; Ueno, T.; Uchiyama, S.; Toda, T.; Uehara, M.; Ishimi, Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: A double-blind, randomized, controlled trial. Menopause 2007, 14, 866–874. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Ho, S.C.; Chen, Y.-M.; Woo, J. A Six-Month Randomized Controlled Trial of Whole Soy and Isoflavones Daidzein on Body Composition in Equol-Producing Postmenopausal Women with Prehypertension. J. Obes. 2013, 2013, 359763. [Google Scholar] [CrossRef] [Green Version]
- Legette, L.L.; Prasain, J.; King, J.; Arabshahi, A.; Barnes, S.; Weaver, C.M. Pharmacokinetics of Equol, a Soy Isoflavone Metabolite, Changes with the Form of Equol (Dietary versus Intestinal Production) in Ovariectomized Rats. J. Agric. Food Chem. 2014, 62, 1294–1300. [Google Scholar] [CrossRef]
- Roberts, M.S.; Magnusson, B.M.; Burczynski, F.J.; Weiss, M. Enterohepatic Circulation: Physiological, pharmacokinetic and clinical implications. Clin. Pharmacokinet. 2002, 41, 751–790. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, M.; Mizushige, T.; Bhattarai, K.; Adhikari, S.; Ishikawa, J.; Kishida, T. Dietary daidzein induces accumulation of S-equol in enterohepatic circulation to far higher levels than that of daidzein in female rats with and without ovariectomy. Biomed. Res. 2019, 40, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef]
- Fujitani, M.; Mizushige, T.; Bhattarai, K.; Iwahara, A.; Aida, R.; Kishida, T. The daidzein- and estradiol- induced anorectic action in CCK or leptin receptor deficiency rats. Biosci. Biotechnol. Biochem. 2015, 79, 1164–1171. [Google Scholar] [CrossRef]
- Polson, D.A.; Thompson, M.P. Macronutrient composition of the diet differentially affects leptin and adiponutrin mRNA expression in response to meal feeding. J. Nutr. Biochem. 2004, 15, 242–246. [Google Scholar] [CrossRef]
- Reiter, A.K.; Crozier, S.J.; Kimball, S.R.; Jefferson, L.S. Meal Feeding Alters Translational Control of Gene Expression in Rat Liver. J. Nutr. 2005, 135, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Vary, T.C.; Lynch, C.J. Meal Feeding Stimulates Phosphorylation of Multiple Effector Proteins Regulating Protein Synthetic Processes in Rat Hearts. J. Nutr. 2006, 136, 2284–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujitani, M.; Mizushige, T.; Bhattarai, K.; Iwahara, A.; Aida, R.; Segawa, T.; Kishida, T. Dynamics of appetite-mediated gene expression in daidzein-fed female rats in the meal-feeding method. Biosci. Biotechnol. Biochem. 2015, 79, 1342–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaszuk, A.; Simpson, C.; Williams, G. Neuropeptide Y, the Hypothalamus and the Regulation of Energy Homeostasis. Horm. Res. Paediatr. 1996, 46, 53–58. [Google Scholar] [CrossRef]
- Gehlert, D.R. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides 1999, 33, 329–338. [Google Scholar] [CrossRef]
- Sawchenko, P.E.; Pfeiffer, S.W. Ultrastructural localization of neuropeptide Y and galanin immunoreactivity in the paraventricular nucleus of the hypothalamus in the rat. Brain Res. 1988, 474, 231–245. [Google Scholar] [CrossRef]
- Horvath, T.L.; Naftolin, F.; Leranth, C.; Sahu, A.; Kalra, S.P. Morphological and pharmacological evidence for neuropeptide Y-galanin interaction in the rat hypothalamus. Endocrinology 1996, 137, 3069–3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Pu, S.; Kalra, P.S.; Hyde, J.F.; Crowley, W.R.; Kalra, S.P. An interactive physiological role of neuropeptide Y and galanin in pulsatile pituitary luteinizing hormone secretion. Endocrinology 1996, 137, 5297–5302. [Google Scholar] [CrossRef]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.-C.; Lin, S.-H.; Hidayah, K.; Lin, C.-I. Equol Pretreatment Protection of SH-SY5Y Cells against Aβ (25–35)-Induced Cytotoxicity and Cell-Cycle Reentry via Sustaining Estrogen Receptor Alpha Expression. Nutrients 2019, 11, 2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Deng, X.; Ma, Z.; Wang, Y. Equol protects PC12 neuronal cells against hypoxia/reoxygenation injury in vitro by reducing reactive oxygen species production. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2016, 36, 1–7. [Google Scholar]
- Bertrand, S.J.; Hu, C.; Aksenova, M.V.; Mactutus, C.F.; Booze, R.M. HIV-1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol. Front. Microbiol. 2015, 6, 894. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, L.; Mao, Z.; Chen, S.; Wong, K.C.; To, J.; Brinton, R.D. Potentiation of brain mitochondrial function by S-equol and R/S-equol estrogen receptor β-selective phytoSERM treatments. Brain Res. 2013, 1514, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol Microbial Metabolites Exhibit Gut and Blood–Brain Barrier Permeability and Protect Murine Microglia against LPS-Induced Inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Bickel, U.; Schumacher, O.P.; Kang, Y.S.; Voigt, K. Poor permeability of morphine 3-glucuronide and morphine 6-glucuronide through the blood-brain barrier in the rat. J. Pharmacol. Exp. Ther. 1996, 278, 107–113. [Google Scholar]
- Wu, D.; Kang, Y.S.; Bickel, U.; Pardridge, W.M. Blood-brain barrier permeability to morphine-6-glucuronide is markedly reduced compared with morphine. Drug Metab. Dispos. 1997, 25, 768–771. [Google Scholar]
- Chang, H.C.; Churchwell, M.I.; Delclos, K.B.; Newbold, R.R.; Doerge, D.R. Mass Spectrometric Determination of Genistein Tissue Distribution in Diet-Exposed Sprague-Dawley Rats. J. Nutr. 2000, 130, 1963–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J. Mechanisms of Action of the Implantable Gastric Stimulator for Obesity. Obes. Surg. 2004, 14 (Suppl. S1), S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Oesch, S.; Rüegg, C.; Fischer, B.; Degen, L.; Beglinger, C. Effect of gastric distension prior to eating on food intake and feelings of satiety in humans. Physiol. Behav. 2006, 87, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Powley, T.L. Gastric volume rather than nutrient content inhibits food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996, 271, R766–R769. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Castellanos, V.H.; Halford, J.C.; Kilara, A.; Panyam, D.; Pelkman, C.L.; Smith, G.P.; Thorwart, M.L. Volume of food consumed affects satiety in men. Am. J. Clin. Nutr. 1998, 67, 1170–1177. [Google Scholar] [CrossRef]
- Adhikari, S.; Bhattarai, K.; Abe, Y.; Kira, M.; Fujitani, M.; Miyada, T.; Kishida, T. Dietary daidzein decreases food intake accompanied with delayed gastric emptying in ovariectomized rats. Biosci. Biotechnol. Biochem. 2020, 84, 1232–1238. [Google Scholar] [CrossRef]
- Nakade, Y.; Tsukamoto, K.; Pappas, T.N.; Takahashi, T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res. 2006, 1111, 117–121. [Google Scholar] [CrossRef]
- Heilig, M. The NPY system in stress, anxiety and depression. Neuropeptides 2004, 38, 213–224. [Google Scholar] [CrossRef]
- Ishiguchi, T.; Nakajima, M.; Sone, H.; Tada, H.; Kumagai, A.K.; Takahashi, T. Gastric distension-induced pyloric relaxation: Central nervous system regulation and effects of acute hyperglycaemia in the rat. J. Physiol. 2001, 533, 801–813. [Google Scholar] [CrossRef]
- Schwen, R.J.; Nguyen, L.; Jackson, R.L. Elucidation of the metabolic pathway of S-equol in rat, monkey and man. Food Chem. Toxicol. 2012, 50, 2074–2083. [Google Scholar] [CrossRef]
- Gardana, C.; Simonetti, P. Long-term kinetics of daidzein and its main metabolites in human equol-producers after soymilk intake: Identification of equol-conjugates by UPLC-orbitrap-MS and influence of the number of transforming bacteria on plasma kinetics. Int. J. Food Sci. Nutr. 2016, 68, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Li, H. Meal-Sensing Signaling Pathways in Functional Dyspepsia. Front. Syst. Neurosci. 2018, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.B.; De Lartigue, G.; Page, A.J. Dissecting the Role of Subtypes of Gastrointestinal Vagal Afferents. Front. Physiol. 2020, 11, 643. [Google Scholar] [CrossRef]
- Gunal, O.; Bozkurt, A.; Deniz, M.; Sungur, M.; Yeg, B.C. Effect of sex steroids on colonic distension-induced delay of gastric emptying in rats. J. Gastroenterol. Hepatol. 2004, 19, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, J.C.; Sampath, C.; Gangula, P.R. Supplementation of 17β-Estradiol Normalizes Rapid Gastric Emptying by Restoring Impaired Nrf2 and nNOS Function in Obesity-Induced Diabetic Ovariectomized Mice. Antioxidants 2020, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, G.; Li, S.; Luu-The, V.; Labrie, F. Oestrogenic Regulation of Pro-Opiomelanocortin, Neuropeptide Y and Corticotrophin-Releasing Hormone mRNAs in Mouse Hypothalamus. J. Neuroendocr. 2007, 19, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.F.; Baker, B.I.; Levy, A.; Wilson, C.A. The Influence of Gonadal Steroids on Pre-Pro Melanin-Concentrating Hormone mRNA in Female Rats. J. Neuroendocr. 2008, 12, 53–59. [Google Scholar] [CrossRef]
- Brown, L.M.; Clegg, D.J. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 2010, 122, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, Y.; Mabuchi, K.; Takano, A.; Hara, Y.; Negishi, H.; Morimoto, K.; Ueno, T.; Uchiyama, S.; Takamata, A. S-equol Exerts Estradiol-Like Anorectic Action with Minimal Stimulation of Estrogen Receptor-α in Ovariectomized Rats. Front. Endocrinol. 2017, 8, 281. [Google Scholar] [CrossRef] [Green Version]
- Sites, C.K.; Cooper, B.C.; Toth, M.J.; Gastaldelli, A.; Arabshahi, A.; Barnes, S. Effect of a daily supplement of soy protein on body composition and insulin secretion in postmenopausal women. Fertil. Steril. 2007, 88, 1609–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maesta, N.; Nahas, E.A.; Nahas-Neto, J.; Orsatti, F.L.; Fernandes, C.E.; Traiman, P.; Burini, R.C. Effects of soy protein and resistance exercise on body composition and blood lipids in postmenopausal women. Maturitas 2007, 56, 350–358. [Google Scholar] [CrossRef]
- Weickert, M.O.; Reimann, M.; Otto, B.; Hall, W.L.; Vafeiadou, K.; Hallund, J.; Ferrari, M.; Talbot, D.; Branca, F.; Bügel, S.; et al. Soy isoflavones increase preprandial peptide YY (PYY), but have no effect on ghrelin and body weight in healthy postmenopausal women. J. Negat. Results Biomed. 2006, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, K.D.R.; Zhao, X.; Shoaf, S.E.; Ragland, K. The Pharmacokinetics of S-(-)Equol Administered as SE5-OH Tablets to Healthy Postmenopausal Women. J. Nutr. 2009, 139, 2037–2043. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, G.; Zhao, L.; Franke, A.A.; Chen, Y.-L.; Mack, W.J.; Brinton, R.D.; Schneider, L.S. Pharmacokinetics and safety profile of single-dose administration of an estrogen receptor β-selective phytoestrogenic (phytoSERM) formulation in perimenopausal and postmenopausal women. Menopause 2018, 25, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Nikander, E.; Tiitinen, A.; Laitinen, K.; Tikkanen, M.; Ylikorkala, O. Effects of Isolated Isoflavonoids on Lipids, Lipoproteins, Insulin Sensitivity, and Ghrelin in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2004, 89, 3567–3572. [Google Scholar] [CrossRef]




| Suggested Mechanisms | References |
|---|---|
| The accumulation of equol in enterohepatic circulation is needed to exert the anorectic effect caused by dietary daidzein. | [42,85,86] |
| Equol alters expression of hypothalamic appetite-related factors. | [48,81] |
| Equol induces the anorectic effect by delaying gastric emptying in OVX rats. | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujitani, M.; Mizushige, T.; Adhikari, S.; Bhattarai, K.; Kishida, T. Mechanism of Soy Isoflavone Daidzein-Induced Female-Specific Anorectic Effect. Metabolites 2022, 12, 252. https://doi.org/10.3390/metabo12030252
Fujitani M, Mizushige T, Adhikari S, Bhattarai K, Kishida T. Mechanism of Soy Isoflavone Daidzein-Induced Female-Specific Anorectic Effect. Metabolites. 2022; 12(3):252. https://doi.org/10.3390/metabo12030252
Chicago/Turabian StyleFujitani, Mina, Takafumi Mizushige, Sudhashree Adhikari, Keshab Bhattarai, and Taro Kishida. 2022. "Mechanism of Soy Isoflavone Daidzein-Induced Female-Specific Anorectic Effect" Metabolites 12, no. 3: 252. https://doi.org/10.3390/metabo12030252