Diffusion Bonding of 9Cr Martensitic/Ferritic Heat-Resistant Steels with an Electrodeposited Ni Interlayer
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
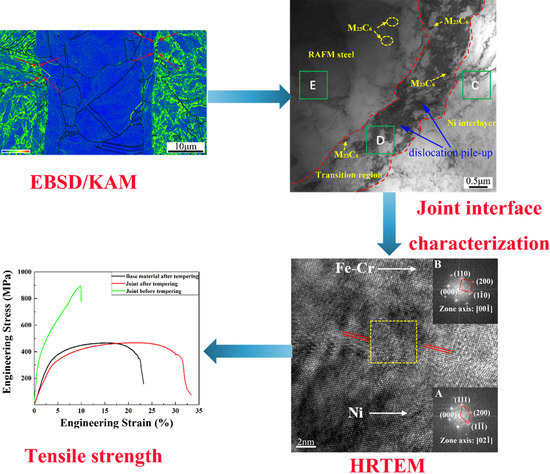
3.1. Microstructural Observation
3.2. Tensile Tests of Joints
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pan, C.H. The International Thermonuclear Experimental Reactor and the future of nuclear fusion energy. Wuli 2010, 39, 375–378. [Google Scholar]
- Klueh, R.L.; Nelson, A.T. Ferritic/martensitic steels for next-generation reactors. J. Nucl. Mater. 2007, 371, 37–52. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C.; Yu, L.; Liu, Y.; Li, H. Phase Transformation Behavior and Microstructural Control of High-Cr Martensitic/Ferritic Heat-resistant Steels for Power and Nuclear Plants: A Review. J. Mater. Sci. Technol. 2015, 31, 235–242. [Google Scholar] [CrossRef]
- Muroga, T.; Gasparotto, M.; Zinkle, S.J. Overview of materials research for fusion reactors. Fusion Eng. Des. 2002, 61, 13–25. [Google Scholar] [CrossRef]
- Baluc, N.; Gelles, D.S.; Jitsukawa, S.; Kimura, A.; Klueh, R.L.; Odette, G.R.; Schaaf, B.V.D.; Yu, J. Status of reduced activation ferritic/martensitic steel development. J. Nucl. Mater. 2007, 367, 33–41. [Google Scholar] [CrossRef]
- Mao, C.; Liu, C.; Yu, L.; Li, H.; Liu, Y. Mechanical properties and tensile deformation behavior of a reduced activated ferritic-martensitic (RAFM) steel at elevated temperatures. Mater. Sci. Eng. A 2018, 725, 283–289. [Google Scholar] [CrossRef]
- Tanigawa, H.; Gaganidze, E.; Hirose, T.; Ando, M.; Zinkle, S.J.; Lindau, R.; Diegele, E. Development of benchmark reduced activation ferritic/martensitic steels for fusion energy applications. Nucl. Fusion 2017, 57. [Google Scholar] [CrossRef]
- Aubert, P.; Tavassoli, F.; Rieth, M.; Diegele, E.; Poitevin, Y. Review of candidate welding processes of RAFM steels for ITER test blanket modules and DEMO. J. Nucl. Mater. 2011, 417, 43–50. [Google Scholar] [CrossRef]
- Hirose, T.; Shiba, K.; Ando, M.; Enoeda, M.; Akiba, M. Joining technologies of reduced activation ferritic/martensitic steel for blanket fabrication. Fusion Eng. Des. 2006, 81, 645–651. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.Y.; Choi, B.K.; Hong, B.G.; Jung, K.J.; Jeong, Y.H. HIP joining of RAFM/RAFM steel and beryllium/RAFM steel for fabrication of the ITER TBM first wall. Met. Mater. Int. 2009, 15, 465–470. [Google Scholar] [CrossRef]
- Ku, D.Y.; Oh, S.; Ahn, M.-Y.; Yu, I.-K.; Kim, D.-H.; Cho, S.; Choi, I.-S.; Kwon, K.-B. TIG and HIP joining of Reduced Activation Ferrite/Martensitic steel for the Korean ITER–TBM. J. Nucl. Mater. 2011, 417, 67–71. [Google Scholar] [CrossRef]
- Francis, J.A.; Mazur, W.; Bhadeshia, H.K.D.H. Review Type IV cracking in ferritic power plant steels. Mater. Sci. Technol.-Lond. 2006, 22, 1387–1395. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.Q.; Li, H. Diffusion behavior at void tip and its contributions to void shrinkage during solid-state bonding. J. Mater. Sci. Technol. 2018, 34, 1449–1454. [Google Scholar] [CrossRef]
- Zhou, X.S.; Liu, Y.C.; Yu, L.M.; Liu, C.X.; Sui, G.F.; Yang, J.G. Uniaxial diffusion bonding of CLAM/CLAM steels: Microstructure and mechanical performance. J. Nucl. Mater. 2015, 461, 301–307. [Google Scholar] [CrossRef]
- Kazakov, N. Diffusion Bonding of Materials; Pergamon: Oxford, UK, 1985. [Google Scholar]
- Haneklaus, N.; Reuven, R.; Cionea, C.; Hosemann, P.; Peterson, P.F. Development of engineering parameters for low pressure diffusion bonds of 316 ss tube-to-tube sheet joints for FHR heat exchangers. In Proceedings of the TMS 2016 145th Annual Meeting & Exhibition, Nashville, TN, USA, 15–17 February 2016; pp. 583–588. [Google Scholar]
- Reuven, R.; Bolind, A.M.; Haneklaus, N.; Cionea, C.; Andreades, C.; Buster, G.; Hosemann, P.; Peterson, P.F. Ni interlayer to improve low pressure diffusion bonding of 316L ss press fit tube-to-tubesheet joints for coiled tube gas heaters. ASME J. Nucl. Radiat. Sci. 2017, 3, 030913. [Google Scholar] [CrossRef]
- Haneklaus, N.; Reuven, R.; Cionea, C.; Hosemann, P.; Peterson, P.F. Tube expansion and diffusion bonding of 316 L stainless steel tube-to-tube sheet joints using a commercial roller tube expander. J. Mater. Process. Technol. 2016, 234, 27–32. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Paul, B.K. Asme Application of Nickel Nanoparticles in Diffusion Bonding of Stainless Steel Surfaces; American Society Mechanical Engineers: New York, NY, USA, 2009; pp. 441–446. [Google Scholar]
- Zhou, X.S.; Dong, Y.T.; Liu, C.X.; Liu, Y.C.; Yu, L.M.; Chen, J.G.; Li, H.J.; Yang, J.G. Transient liquid phase bonding of CLAM/CLAM steels with Ni-based amorphous foil as the interlayer. Mater. Des. 2015, 88, 1321–1325. [Google Scholar] [CrossRef]
- Zhong, Z.; Jung, H.; Hinoki, T.; Kohyama, A. Effect of joining temperature on the microstructure and strength of tungsten/ferritic steel joints diffusion bonded with a nickel interlayer. J. Mater. Process. Technol. 2010, 210, 1805–1810. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.H.; Hinoki, T.; Kohyama, A. Effect of holding time on the microstructure and strength of tungsten/ferritic steel joints diffusion bonded with a nickel interlayer. Mater. Sci. Eng. A 2009, 518, 167–173. [Google Scholar] [CrossRef]
- Kundu, S.; Chatterjee, S. Interfacial microstructure and mechanical properties of diffusion-bonded titanium–stainless steel joints using a nickel interlayer. Mater. Sci. Eng. A 2006, 425, 107–113. [Google Scholar] [CrossRef]
- Abe, F. Precipitate design for creep strengthening of 9% Cr tempered martensitic steel for ultra-supercritical power plants. Sci. Technol. Adv. Mater. 2008, 9, 013002. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Liu, Y.; Yan, B.; Li, H. Effects of tantalum content on the microstructure and mechanical properties of low-carbon RAFM steel. J. Nucl. Mater. 2016, 479, 295–301. [Google Scholar] [CrossRef]
- Chen, J.G.; Liu, Y.C.; Xiao, Y.T.; Liu, Y.H.; Liu, C.X.; Li, H.J. Improvement of High-Temperature Mechanical Properties of Low-Carbon RAFM Steel by MX Precipitates. Acta Metall. Sin.-Engl. Lett. 2018, 31, 706–712. [Google Scholar] [CrossRef]
- Yan, B.Y.; Liu, Y.C.; Wang, Z.J.; Liu, C.X.; Si, Y.H.; Li, H.J.; Yu, J.X. The Effect of Precipitate Evolution on Austenite Grain Growth in RAFM Steel. Materials 2017, 10, 1017. [Google Scholar] [CrossRef]
- Rabkin, E.; Klinger, L.; Izyumova, T.; Semenov, V.N. Diffusion-induced grain boundary porosity in NiAl. Scr. Mater. 2000, 42, 1031–1037. [Google Scholar] [CrossRef]
- Balluffi, R.W. Grain boundary diffusion mechanisms in metals. Metall. Trans. B 1982, 13, 527–553. [Google Scholar] [CrossRef]
- Yu, J.Q.; Yi, W.Z.; Chen, B.D.; Chen, H.J. Phase Diagram of Binary Alloys; University of Shanghai for Science and Technology: Shanghai, China, 1987. [Google Scholar]
- Peng, K.P.; Qian, K.W.; Chen, W.Z. Effect of dynamic strain aging on high temperature properties of austenitic stainless steel. Mater. Sci. Eng. A 2004, 379, 372–377. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Miyamoto, G.; Shinbo, K.; Furuhara, T. Effects of α/γ orientation relationship on VC interphase precipitation in low-carbon steels. Scr. Mater. 2013, 69, 17–20. [Google Scholar] [CrossRef]
- Kitahara, H.; Ueji, R.; Tsuji, N.; Minamino, Y. Crystallographic features of lath martensite in low-carbon steel. Acta Mater. 2006, 54, 1279–1288. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, D.; Qi, X.; Jiang, Z.; Zhang, H. Microstructure and mechanical properties of Cr14 ultra-high-strength steel at different tempering temperatures around 773 K. Mater. Sci. Eng. A 2017, 698, 152–161. [Google Scholar] [CrossRef]
- Shirazi, H.; Miyamoto, G.; Nedjad, S.H.; Chiba, T.; Ahmadabadi, M.N.; Furuhara, T. Microstructure evolution during austenite reversion in Fe-Ni martensitic alloys. Acta Mater. 2018, 144, 269–280. [Google Scholar] [CrossRef]
- Shi, X.; Zeng, W.; Zhao, Q.; Peng, W.; Kang, C. Study on the microstructure and mechanical properties of Aermet 100 steel at the tempering temperature around 482 °C. J. Alloys Compd. 2016, 679, 184–190. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Yang, Z.; Su, J.; Weng, Y. Analysis of fracture toughness in high Co–Ni secondary hardening steel using FEM. Mater. Sci. Eng. A 2015, 646, 1–7. [Google Scholar] [CrossRef]
- Zhou, H.W.; Fang, J.F.; Chen, Y.; Yang, L.; Zhang, H.; Lu, Y.; He, Y.Z. Internal friction studies on dynamic strain aging in P91 ferritic steel. Mater. Sci. Eng. A 2016, 676, 361–365. [Google Scholar] [CrossRef]











| Element | C | N | Cr | W | Mn | Si | V | Ta | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.04 | 0.02 | 8.93 | 1.71 | 0.44 | 0.04 | 0.22 | 0.073 | Bal. |
| Specimen No. | Chemical and Parameters | Data |
|---|---|---|
| 1 | Nickel sulfate | 240 g/L |
| 2 | Nickel chloride | 40 g/L |
| 3 | Boric acid | 40 g/L |
| 4 | Ammonium chloride | 40 g/L |
| 5 | Lauryl sodium sulfate | 0.15 g/L |
| 6 | Time duration | 60 min |
| 7 | Temperature | 35 °C |
| 8 | Current density | 3 A/dm2 |
| 9 | pH value | 5.8 |
| Region | A | B | C | D | E | |
|---|---|---|---|---|---|---|
| Element | ||||||
| Fe | 50.28 | 90.26 | 51.27 | 81.26 | 90.13 | |
| Ni | 40.34 | 0.7 | 39.64 | 9.51 | 0.57 | |
| Cr | 4.86 | 8.33 | 5.47 | 7.85 | 8.85 | |
| W | 4.52 | 4.5 | 3.60 | 1.35 | 0.43 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, Z.; Liu, Y.; Li, W.; Liu, C.; Li, H. Diffusion Bonding of 9Cr Martensitic/Ferritic Heat-Resistant Steels with an Electrodeposited Ni Interlayer. Metals 2018, 8, 1012. https://doi.org/10.3390/met8121012
Gao Y, Wang Z, Liu Y, Li W, Liu C, Li H. Diffusion Bonding of 9Cr Martensitic/Ferritic Heat-Resistant Steels with an Electrodeposited Ni Interlayer. Metals. 2018; 8(12):1012. https://doi.org/10.3390/met8121012
Chicago/Turabian StyleGao, Yan, Zumin Wang, Yongchang Liu, Wenchao Li, Chenxi Liu, and Huijun Li. 2018. "Diffusion Bonding of 9Cr Martensitic/Ferritic Heat-Resistant Steels with an Electrodeposited Ni Interlayer" Metals 8, no. 12: 1012. https://doi.org/10.3390/met8121012