Copper and Cyanide Extraction with Emulsion Liquid Membrane with LIX 7950 as the Mobile Carrier: Part 1, Emulsion Stability
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Procedure
2.3. Analysis and Calculation
- δ: the apparent leakage (breakage) of ELMs, %;
- CKR: the molar concentration of K+ in the raffinate, mol/L;
- CKi: the molar concentration of K+ in the initial internal phase, mol/L;
- VR: the volume of the raffinate, L;
- Vi: the volume of the initial internal phase, L;
- VE: the apparent volume of emulsions before contacting the feed, L;
- : the apparent volume of emulsions after extraction, L;
- Vf: the initial volume of feed, L;
- E: the removal (or extraction) of the target species from the feed solution by ELMs, %;
- Cf: the molar concentration of the target species in the feed, mol/L;
- CR: the molar concentration of the target species in the raffinate, mol/L;
3. Results and Discussion
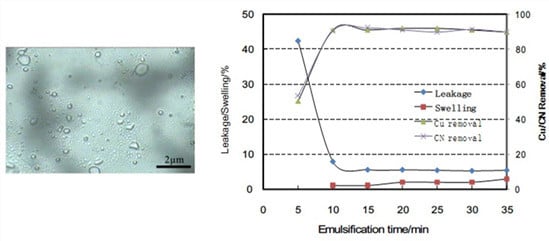
3.1. Influence of Mixing for ELM Formation



3.2. Influence of KOH in the Internal Phase

3.3. Influence of Extractant and Surfactant


3.4. Influence of Volume Ratio of Membrane to Internal Phases
| Vm/Vint | Leakage, % | Swelling, % | Cuext, mg/L | CNext, mg/L | Cu Removal, % | CN Removal, % |
|---|---|---|---|---|---|---|
| 1:3 | 12.5 | 1 | 4.3 | 5.1 | 78.0 | 78.2 |
| 1:2 | 2.5 | 2 | 0.9 | 1.2 | 95.5 | 94.9 |
| 1:1 | 3 | 2 | 1.7 | 2.2 | 91.0 | 90.8 |
| 2:1 | 4 | 8 | 10.8 | 13.0 | 44.3 | 45.0 |
| 3:1 | 15 | 10 | 11.7 | 13.9 | 39.3 | 41.2 |
| 4:1 | 18 | 12 | 11.3 | 13.3 | 41.3 | 43.8 |
| 5:1 | 28 | 18 | 10.8 | 13.2 | 44.0 | 44.0 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marsden, J.; House, I. Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2006; pp. 15–45. [Google Scholar]
- DeVries, F. Brief overview of the Baia Mare Dam Breach. In Cyanide: Social, Industrial and Economic Aspects; Young, A.A., Twidwell, L.G., Anderson, C.G., Eds.; TMS: Warrendale, PA, USA, 2001; pp. 11–14. [Google Scholar]
- Fleming, C.A. Cyanide Recovery. In Advances in Gold Ore Processing; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 703–727. [Google Scholar]
- Jay, W.H. Copper Cyanide Recovery System. In Cyanide: Social, Industrial and Economic Aspects; Young, A.A., Twidwell, L.G., Anderson, C.G., Eds.; TMS: Warrendale, PA, USA, 2001; pp. 317–340. [Google Scholar]
- Botz, M.M.; Mudder, T.I.; Akcil, A.U. Cyanide Treatment: Physical, Chemical and Biological Process. In Advances in Gold Ore Processing; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 672–702. [Google Scholar]
- Barter, J.; Lane, G.; Mitchell, D.; Kelson, R.; Dunne, R.; Trang, C.; Dreisinger, D. Cyanide Management by SART. In Cyanide: Social, Industrial and Economic Aspects; Young, A.A., Twidwell, L.G., Anderson, C.G., Eds.; TMS: Warrendale, PA, USA, 2001; pp. 549–562. [Google Scholar]
- Adams, M.D. Removal of cyanide from solution using activated carbon. Miner. Eng. 1994, 7, 1165–1177. [Google Scholar] [CrossRef]
- Goldblatt, E. Recovery of cyanide from waste cyanide solution by ion exchange. Ind. Eng. Chem. 1956, 48, 2107–2114. [Google Scholar] [CrossRef]
- Le Vier, K.M.; Fitzpatrick, T.A.; Brunk, K.A.; Ellett, W.N. AuGMENT Technologies: An update. In Proceedings of the Randol Gold Forum, Monterey, CA, USA, 18–21 May 1997; pp. 135–137.
- Leão, V.A.; Ciminelli, V.S.T.; Costa, R.S. Cyanide recycling using strong-base ion exchange resins. JOM 1998, 50, 66–69. [Google Scholar] [CrossRef]
- Davis, M.R.; MacKenzie, M.W.; Sole, K.C.; Virnig, M.J. A proposed solvent extraction route for the treatment of copper cyanide solutions produced in leaching of gold ores. In Proceedings of the Alta Copper Hydrometallurgy Forum, Brisbane, Australia, 20–21 October 1998; pp. 42–56.
- Xie, F.; Dreisinger, D. Copper solvent extraction from waster cyanide solution by a solvent mixture of a quaternary amine and nonylphenol. In Proceedings of the ISEC 2008, Tucson, AZ, USA, 15–19 September 2008; Moyer, B.A., Ed.; CIM: Tuscon, AZ, USA, 2008; pp. 107–112. [Google Scholar]
- Xie, F.; Dreisinger, D. Recovery of copper cyanide from waste cyanide solution by LIX 7950. Miner. Eng. 2009, 22, 190–195. [Google Scholar] [CrossRef]
- Riveros, P.A. Studies on the solvent extraction of gold from cyanide media. Hydrometallurgy 1990, 24, 135–156. [Google Scholar] [CrossRef]
- Flynn, C.M.; Sandra, L.M. Cyanide Chemistry—Precious Metals Processing and Waste Treatment; Information Circular (United States Bureau of Mines) 9429; United States Department of Interior: Washington, DC, USA, 1995; pp. 1–28. [Google Scholar]
- Bodzek, M. Membrane Technologies for the Removal of Micropollutants in Water Treatment. In Advances in Membrane Technologies for Water Treatment: Materials, Processes and Applications; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Elsevier: Cambridge, UK, 2015; pp. 465–517. [Google Scholar]
- Li, N.N.; Chan, R.P.; Naden, D.; Lai, R.W.M. Liquid membrane processes for copper extraction. Hydrometallurgy 1983, 9, 277–305. [Google Scholar] [CrossRef]
- Li, Q.M.; Liu, Q.; Wei, X. Separation study of mercury through an emulsion liquid membrane. Talanta 1996, 43, 1837–1842. [Google Scholar] [CrossRef]
- Kumbasar, R. Selective extraction of cobalt from strong acidic solutions containing cobalt and nickel through emulsion liquid membrane using TIOA as carrier. J. Ind. Eng. Chem. 2012, 18, 2076–2082. [Google Scholar] [CrossRef]
- Pabby, A.K.; Sastre, A.M. State-of-the-art review on hollow fibre contactor technology and membrane-based extraction processes. J. Membr. Sci. 2013, 430, 263–303. [Google Scholar] [CrossRef]
- García, M.G.; Acosta, A.O.; Marchese, J. Emulsion liquid membrane pertraction of Cr(III) from aqueous solutions using PC-88A as carrier. Desalination 2013, 318, 88–96. [Google Scholar] [CrossRef]
- Martin, T.P.; Davies, G.A. The extraction of copper from dilute aqueous solutions using a liquid membrane process. Hydrometallurgy 1977, 2, 315–334. [Google Scholar] [CrossRef]
- Kitagawa, T.; Nishikawa, Y.; Frankenfeld, J.; Li, N.N. Waste water treatment by liquid membrane process. Environ. Sci. Technol. 1977, 11, 602–605. [Google Scholar] [CrossRef]
- Chakraborty, M.; Bhattacharya, C.; Datta, S. Emulsion Liquid Membranes: Definitions and Classification, Theories, Module Design, Applications New Directions and Perspectives. In Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment; Kislik, V.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 141–199. [Google Scholar]
- Marr, R.J.; Draxler, J. Emulsion Liquid Membrane Application. In Membrane Handbook; Ho, W.S., Sirkar, K.K., Eds.; Chapman & Hall: New York, NY, USA, 1992; pp. 701–717. [Google Scholar]
- Chiha, M.; Hamdaoui, O.; Ahmedchekkat, F.; Petrier, C. Study on ultrasonically assisted emulsification and recovery of copper(II) from wastewater using an emulsion liquid membrane process. Ultrason. Sonochem. 2010, 17, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kumbasar, R.A. Selective separation of chromium(VI) from acidic solutions containing various metal ions through emulsion liquid membrane using trioctylamine as extractant. Sep. Purif. Technol. 2008, 64, 56–62. [Google Scholar] [CrossRef]
- Aydiner, C.; Kobya, M.; Demirbas, E. Cyanide ions transport from aqueous solutions by using quaternary ammonium salts through bulk liquid membranes. Desalination 2005, 180, 139–150. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M.; Sastre, A.M. Facilitated supported liquid membrane transport of gold(I) and gold(III) using Cyanex 921. J. Membr. Sci. 2005, 252, 237–244. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M. Transport of Au(CN)2− across a supported liquid membrane using mixtures of amine Primene JMT and phosphine oxide Cyanex 923. Hydrometallurgy 2004, 74, 157–163. [Google Scholar] [CrossRef]
- Pabby, A.K.; Haddad, R.; Alguacil, F.J.; Sastre, A.M. Improved kinetics-based gold cyanide extraction with mixture of LIX79 + TOPO utilizing hollow fiber membrane contactors. Chem. Eng. J. 2004, 100, 11–22. [Google Scholar]
- Kumar, A.; Haddad, R.; Benzal, G.; Ninou, R.; Sastre, A.M. Use of modified membrane carrier system for recovery of gold cyanide from alkaline cyanide media using hollow fiber supported liquid membranes: Feasibility studies and mass transfer modeling. J. Membr. Sci. 2000, 174, 17–30. [Google Scholar] [CrossRef]
- León, G.; Guzmán, M.A. Kinetic study of the effect of carrier and stripping agent concentrations on the facilitated transport of cobalt through bulk liquid membranes. Desalination 2005, 184, 79–87. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kathiresan, M. Extraction of metal ions by emulsion liquid membrane using bi-functional surfactant: Equilibrium and kinetic studies. Sep. Purif. Technol. 2000, 19, 3–9. [Google Scholar] [CrossRef]
- Singh, R.; Mehta, R.R.; Kumar, V. Simultaneous removal of copper, nickel and zinc metal ions using bulk liquid membrane system. Desalination 2011, 272, 170–173. [Google Scholar] [CrossRef]
- Ahmada, A.L.; Kusumastuti, A.; Dereka, C.J.C.; Ooi, B.S. Emulsion liquid membrane for heavy metal removal: An overview on emulsion stabilization and destabilization. Chem. Eng. J. 2011, 171, 870–882. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Cyanides in Water. In D2036-09, Annual Book of ASTM Standards 2015; ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–20. [Google Scholar]
- Wan, Y.; Zhang, X. Swelling determination of W/O/W emulsion liquid membranes. J. Membr. Sci. 2002, 196, 185–201. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Chang, Y.; Wang, W.; Xie, F.; Asselin, E.; Dreisinger, D. Copper and Cyanide Extraction with Emulsion Liquid Membrane with LIX 7950 as the Mobile Carrier: Part 1, Emulsion Stability. Metals 2015, 5, 2034-2047. https://doi.org/10.3390/met5042034
Lu D, Chang Y, Wang W, Xie F, Asselin E, Dreisinger D. Copper and Cyanide Extraction with Emulsion Liquid Membrane with LIX 7950 as the Mobile Carrier: Part 1, Emulsion Stability. Metals. 2015; 5(4):2034-2047. https://doi.org/10.3390/met5042034
Chicago/Turabian StyleLu, Diankun, Yongfeng Chang, Wei Wang, Feng Xie, Edouard Asselin, and David Dreisinger. 2015. "Copper and Cyanide Extraction with Emulsion Liquid Membrane with LIX 7950 as the Mobile Carrier: Part 1, Emulsion Stability" Metals 5, no. 4: 2034-2047. https://doi.org/10.3390/met5042034