Insights into Advancements and Electrons Transfer Mechanisms of Electrogens in Benthic Microbial Fuel Cells
Abstract
:1. Introduction
2. Benthic Microbial Fuel Cell (BMFC)
3. Degradation of Organic Matter by BMFC
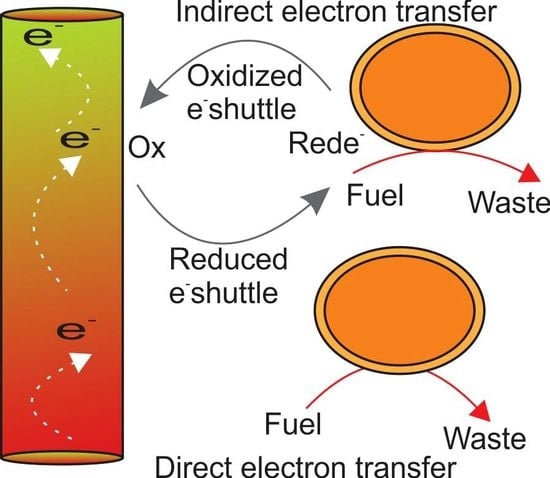
4. Electron Transfer Mechanism by Electrogens
4.1. Direct Electron Transfer
4.2. Indirect Electron Transfer
5. Performance of BMFC Affected by Organic Substrate
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grey, D.; Garrick, D.; Blackmore, D.; Kelman, J.; Muller, M.; Sadoff, C. Water security in one blue planet: Twenty-first century policy challenges for science. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-W.; Yu, H.-Q. Stimulating sediment bioremediation with benthic microbial fuel cells. Biotechnol. Adv. 2015, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E.; Khan, M.M.; Min, B.-K.; Cho, M.H. Microbial fuel cell assisted band gap narrowed TiO2 for visible light-induced photocatalytic activities and power generation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.E.; Han, T.H.; Khan, M.M.; Karim, M.R.; Cho, M.H. Environmentally sustainable fabrication of Ag@g–C3N4 nanostructures and their multifunctional efficacy as antibacterial agents and photocatalysts. ACS Appl. Nano. Mater. 2018, 1, 2912–2922. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Environmentally sustainable biogenic fabrication of AuNP decorated-graphitic gC3N4 nanostructures towards improved photoelectrochemical performances. RSC Adv. 2018, 8, 13898–13909. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, A.A.; Khatoon, A.; Mohd Setapar, S.H.; Umar, K.; Parveen, T.; Mohamad Ibrahim, M.N.; Ahmad, A.; Rafatullah, M. Outlook on the role of microbial fuel cells in remediation of environmental pollutants with electricity generation. Catalysts 2020, 10, 819. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent advances in anodes for microbial fuel cells: An overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Liew, K.B.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Lim, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Nastro, R.A. Enhanced bioremediation of toxic metals and harvesting electricity through sediment microbial fuel cell. Int. J. Energy Res. 2017, 41, 2345–2355. [Google Scholar] [CrossRef]
- Martins, G.; Peixoto, L.; Brito, A.G.; Nogueira, R. Phosphorus–iron interaction in sediments: Can an electrode minimize phosphorus release from sediments? Rev. Environ. Sci. Biotechnol. 2014, 13, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tian, Y.; Qu, Y.; Qiu, Y.; Liu, J.; Feng, Y. A pilot-scale benthic microbial electrochemical system (BMES) for enhanced organic removal in sediment restoration. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Syakir, M.I. The behaviour of membrane less sediment microbial fuel cell in the terms of bioremediation and power generation. Malays. J. Microbiol. 2018, 14, 108–112. [Google Scholar]
- Reimers, C.E.; Girguis, P.; Stecher, H.A.; Tender, L.M.; Ryckelynck, N.; Whaling, P. Microbial fuel cell energy from an ocean cold seep. Geobiology 2006, 4, 123–136. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Reimers, C.E.; White, H.K.; Sharma, S.; Girguis, P.R. Sustainable energy from deep ocean cold seeps. Energy Environ. Sci. 2008, 1, 584–593. [Google Scholar] [CrossRef]
- Hong, S.W.; Chang, I.S.; Choi, Y.S.; Kim, B.H.; Chung, T.H. Responses from freshwater sediment during electricity generation using microbial fuel cells. Bioprocess Biosyst. Eng. 2009, 32, 389–395. [Google Scholar] [CrossRef]
- Rezaei, F.; Richard, T.L.; Brennan, R.A.; Logan, B.E. Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environ. Sci. Technol. 2007, 41, 4053–4058. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.; Zhuang, L. A new approach to in situ sediment remediation based on air-cathode microbial fuel cells. J. Soils. Sediments 2010, 10, 1427–1433. [Google Scholar] [CrossRef]
- Wu, M.; Xu, X.; Lu, K.; Li, X. Effects of the presence of nanoscale zero-valent iron on the degradation of polychlorinated biphenyls and total organic carbon by sediment microbial fuel cell. Sci. Total. Environ. 2019, 656, 39–44. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Syakir, M.I. A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: Mechanisms and future prospective. Int. J. Energy Res. 2017, 41, 1242–1264. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Q.; Wu, M.; Ding, J.; Zhang, W. Biodegradation of organic matter and anodic microbial communities analysis in sediment microbial fuel cells with/without Fe (III) oxide addition. Bioresour. Technol. 2017, 225, 402–408. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Recent progress of metal–graphene nanostructures in photocatalysis. Nanoscale 2018, 10, 9427–9440. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Canales, M.; Kuzume, A.; Borjas, Z.; Füeg, M.; Lovley, D.; Wandlowski, T.; Esteve-Núñez, A. A severe reduction in the cytochrome C content of Geobacter sulfurreducens eliminates its capacity for extracellular electron transfer. Environ. Microbiol. Rep. 2015, 7, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Green synthesis, photocatalytic and photoelectrochemical performance of an Au–Graphene nanocomposite. RSC Adv. 2015, 5, 26897–26904. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Biogenic synthesis of a Ag–graphene nanocomposite with efficient photocatalytic degradation, electrical conductivity and photoelectrochemical performance. New J. Chem. 2015, 39, 8121–8129. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Cao, X.; Wang, Y.; Zou, L.; Ge, X.; Zhao, Y.; Si, Z.; Wang, Y. Chlorella vulgaris on the cathode promoted the performance of sediment microbial fuel cells for electrogenesis and pollutant removal. Sci. Total. Environ. 2020, 728, 138011. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Quan, X.; Chen, L.; Zheng, Y. Application of slow-release carbon sources embedded in polymer for stable and extended power generation in microbial fuel cells. Chemosphere 2020, 244, 125515. [Google Scholar] [CrossRef]
- Song, H.-L.; Zhu, Y.; Li, J. Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells—A mini review. Arab. J. Chem. 2019, 12, 2236–2243. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yang, A. Modelling the impact of operating mode and electron transfer mechanism in microbial fuel cells with two-species anodic biofilm. Biochem. Eng. J. 2020, 107560. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Shakoori, F.R. Electrochemistry and microbiology of microbial fuel cells treating marine sediments polluted with heavy metals. RSC Adv. 2018, 8, 18800–18813. [Google Scholar] [CrossRef] [Green Version]
- Jothinathan, D.; Mylsamy, P.; Bruno, L.B. Electricigens: Role and prominence in microbial fuel cell performance. In Microbial Fuel Cell Technology for Bioelectricity; Springer: Berlin/Heidelberg, Germany, 2018; pp. 169–185. [Google Scholar]
- Tremblay, P.-L.; Angenent, L.T.; Zhang, T. Extracellular electron uptake: Among autotrophs and mediated by surfaces. Trends Biotechnol. 2017, 35, 360–371. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Carbajosa, S.; Malki, M.; Caillard, R.; Lopez, M.F.; Palomares, F.J.; Martín-Gago, J.A.; Rodríguez, N.; Amils, R.; Fernández, V.M.; De Lacey, A.L. Electrochemical growth of Acidithiobacillus ferrooxidans on a graphite electrode for obtaining a biocathode for direct electrocatalytic reduction of oxygen. Biosens. Bioelectron. 2010, 26, 877–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summers, Z.M.; Gralnick, J.A.; Bond, D.R. Cultivation of an obligate Fe (II)-oxidizing lithoautotrophic bacterium using electrodes. mBio 2013, 4, e00420-12. [Google Scholar] [CrossRef] [Green Version]
- Bose, A.; Gardel, E.J.; Vidoudez, C.; Parra, E.; Girguis, P.R. Electron uptake by iron-oxidizing phototrophic bacteria. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Beese-Vasbender, P.F.; Grote, J.-P.; Garrelfs, J.; Stratmann, M.; Mayrhofer, K.J. Selective microbial electrosynthesis of methane by a pure culture of a marine lithoautotrophic archaeon. Bioelectrochemistry 2015, 102, 50–55. [Google Scholar] [CrossRef]
- Aryal, N.; Tremblay, P.-L.; Lizak, D.M.; Zhang, T. Performance of different Sporomusa species for the microbial electrosynthesis of acetate from carbon dioxide. Bioresour. Technol. 2017, 233, 184–190. [Google Scholar] [CrossRef]
- Beese-Vasbender, P.F.; Nayak, S.; Erbe, A.; Stratmann, M.; Mayrhofer, K.J. Electrochemical characterization of direct electron uptake in electrical microbially influenced corrosion of iron by the lithoautotrophic SRB Desulfopila corrodens strain IS4. Electrochim. Acta 2015, 167, 321–329. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Pisciotta, J.M.; Zaybak, Z.; Call, D.F.; Nam, J.-Y.; Logan, B.E. Enrichment of microbial electrolysis cell biocathodes from sediment microbial fuel cell bioanodes. Appl. Environ. Microbiol. 2012, 78, 5212–5219. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-T.; Bai, M.-D.; Wu, S.-I.; Chen, C.-C.; Lu, W.-J.; Wan, H.-P.; Huang, C. Electro-autotrophs induced the growth of exoelectrogens on the anode in a microbial fuel cell. Biochem. Eng. J. 2019, 141, 29–34. [Google Scholar] [CrossRef]
- Peng, T.; Feng, C.; Hu, W.; Chen, N.; He, Q.; Dong, S.; Xu, Y.; Gao, Y.; Li, M. Treatment of nitrate-contaminated groundwater by heterotrophic denitrification coupled with electro-autotrophic denitrifying packed bed reactor. Biochem. Eng. J. 2018, 134, 12–21. [Google Scholar] [CrossRef]
- Jabeen, G.; Farooq, R. Bio-electrochemical synthesis of commodity chemicals by autotrophic acetogens utilizing CO2 for environmental remediation. J. Biosci. 2016, 41, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Zhao, F.; Slade, R.C.T.; Varcoe, J.R. Techniques for the study and development of microbial fuel cells: An electrochemical perspective. Chem. Soc. Rev. 2009, 38, 1926–1939. [Google Scholar] [CrossRef] [Green Version]
- Malvankar, N.S.; Lovley, D.R. Microbial nanowires for bioenergy applications. Curr. Opin. Biotechnol. 2014, 27, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Z.; Rafatullah, M.; Khan, M.A.; Siddiqui, M.R. Bioremediation and Electricity Generation by Using Open and Closed Sediment Microbial Fuel Cells. Front. Microbiol. 2019, 9, 3348. [Google Scholar] [CrossRef] [Green Version]
- Rotaru, A.-E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Link between capacity for current production and syntrophic growth in Geobacter species. Front. Microbiol. 2015, 6, 744. [Google Scholar] [CrossRef] [Green Version]
- Xing, D.; Zuo, Y.; Cheng, S.; Regan, J.M.; Logan, B.E. Electricity generation by Rhodopseudomonas palustris DX-1. Environ. Sci. Technol. 2008, 42, 4146–4151. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl. Environ. Microbiol. 2005, 71, 2186–2189. [Google Scholar] [CrossRef] [Green Version]
- Strycharz, S.M.; Woodard, T.L.; Johnson, J.P.; Nevin, K.P.; Sanford, R.A.; Löffler, F.E.; Lovley, D.R. Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by Geobacter lovleyi. Appl. Environ. Microbiol. 2008, 74, 5943–5947. [Google Scholar] [CrossRef] [Green Version]
- Virdis, B.; Rabaey, K.; Yuan, Z.; Keller, J. Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res. 2008, 42, 3013–3024. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef]
- Wrighton, K.C.; Thrash, J.C.; Melnyk, R.A.; Bigi, J.P.; Byrne-Bailey, K.G.; Remis, J.P.; Schichnes, D.; Auer, M.; Chang, C.J.; Coates, J.D. Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2011, 77, 7633–7639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, D.; LaBelle, E.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1. J. Biol. Chem. 2009, 284, 28865–28873. [Google Scholar] [CrossRef] [Green Version]
- Titov, D.V.; Cracan, V.; Goodman, R.P.; Peng, J.; Grabarek, Z.; Mootha, V.K. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 2016, 352, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Mathis, B.J.; Marshall, C.W.; Milliken, C.E.; Makkar, R.S.; Creager, S.E.; May, H.D. Electricity generation by thermophilic microorganisms from marine sediment. Appl. Microbiol. Biotecnol. 2008, 78, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. The microbe electric: Conversion of organic matter to electricity. Curr. Opin. Biotechnol. 2008, 19, 564–571. [Google Scholar] [CrossRef]
- Zhang, T.; Cui, C.; Chen, S.; Yang, H.; Shen, P. The direct electrocatalysis of Escherichia coli through electroactivated excretion in microbial fuel cell. Electrochem. Commun. 2008, 10, 293–297. [Google Scholar] [CrossRef]
- Sydow, A.; Krieg, T.; Mayer, F.; Schrader, J.; Holtmann, D. Electroactive bacteria—Molecular mechanisms and genetic tools. Appl. Microbiol. Biotechnol. 2014, 98, 8481–8495. [Google Scholar] [CrossRef]
- Wang, Z.; Leary, D.H.; Malanoski, A.P.; Li, R.W.; Hervey, W.J.; Eddie, B.J.; Tender, G.S.; Yanosky, S.G.; Vora, G.J.; Tender, L.M. A previously uncharacterized, nonphotosynthetic member of the Chromatiaceae is the primary CO2-fixing constituent in a self-regenerating biocathode. Appl. Environ. Microbiol. 2015, 81, 699–712. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Gu, T. Bioenergetics explains when and why more severe MIC pitting by SRB can occur. In Proceedings of the NACE International Corrosion 2011 Conference and Expo, Houston, TX, USA, 13–17 March 2011. [Google Scholar]
- Cordas, C.M.; Moura, J.J.G. Sulphate reducing bacteria-electroactive biofilm formation. Int. J. Med. Biol. Front. 2011, 17, 295–312. [Google Scholar]
- Arends, J.B.; Patil, S.A.; Roume, H.; Rabaey, K. Continuous long-term electricity-driven bioproduction of carboxylates and isopropanol from CO2 with a mixed microbial community. J. CO2 Util. 2017, 20, 141–149. [Google Scholar] [CrossRef] [Green Version]
- de Campos Rodrigues, T.; Rosenbaum, M.A. Microbial electroreduction: Screening for new cathodic biocatalysts. ChemElectroChem 2014, 1, 1916–1922. [Google Scholar] [CrossRef]
- Qian, Z.; Tianwei, H.; Mackey, H.R.; van Loosdrecht, M.C.; Guanghao, C. Recent advances in dissimilatory sulfate reduction: From metabolic study to application. Water Res. 2019, 150, 162–181. [Google Scholar] [CrossRef] [PubMed]
- Berlendis, S.; Ranchou-Peyruse, M.; Fardeau, M.-L.; Lascourreges, J.-F.; Joseph, M.; Ollivier, B.; Aüllo, T.; Dequidt, D.; Magot, M.; Ranchou-Peyruse, A. Desulfotomaculum aquiferis sp. nov. and Desulfotomaculum profundi sp. nov., isolated from a deep natural gas storage aquifer. Int. J. Syst. Evol. Microbiol. 2016, 66, 4329–4338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; May, H.D.; Lu, L.; Liang, P.; Huang, X.; Ren, Z.J. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019, 149, 42–55. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, R.J. Bidirectional extracellular electron transfers of electrode-biofilm: Mechanism and application. Bioresour. Technol. 2019, 271, 439–448. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Bilal, M.; Wang, S.; Iqbal, H.M.N.; Zhao, Y.; Hu, H.; Wang, W.; Zhang, X. Metabolic engineering strategies for enhanced shikimate biosynthesis: Current scenario and future developments. Appl. Microbiol. Biotecnol. 2018, 102, 7759–7773. [Google Scholar] [CrossRef]
- Li, Y.; Marshall, A.; Gostomski, P.A. Gaseous pollutant treatment and electricity generation in microbial fuel cells (MFCs) utilising redox mediators. Rev. Environ. Sci. Bio/Technol. 2014, 13, 35–51. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Talebnia, F.; Premier, G.C.; Bakeri, G.; Kim, J.R.; Oh, S.-E. Thionine increases electricity generation from microbial fuel cell using Saccharomyces cerevisiae and exoelectrogenic mixed culture. J. Microbiol. 2012, 50, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Poulsen, F.W.; Min, B.; Angelidaki, I.; Thomsen, A.B. The effect of different substrates and humic acid on power generation in microbial fuel cell operation. Bioresour. Technol. 2009, 100, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Shakeri, M.; Zare, H. Methylene blue as electron promoters in microbial fuel cell. Int. J. Hydrogen Energy 2011, 36, 13335–13341. [Google Scholar] [CrossRef]
- Fernando, E.; Keshavarz, T.; Kyazze, G. Enhanced bio-decolourisation of acid orange 7 by Shewanella oneidensis through co-metabolism in a microbial fuel cell. Int. Biodeterior. Biodegrad. 2012, 72, 1–9. [Google Scholar] [CrossRef]
- Ringeisen, B.R.; Henderson, E.; Wu, P.K.; Pietron, J.; Ray, R.; Little, B.; Biffinger, J.C.; Jones-Meehan, J.M. High Power Density from a Miniature Microbial Fuel Cell Using Shewanella oneidensis DSP10. Environ. Sci. Technol. 2006, 40, 2629–2634. [Google Scholar] [CrossRef] [Green Version]
- Menicucci, J.; Beyenal, H.; Marsili, E.; Veluchamy, R.A.; Demir, G.; Lewandowski, Z. Procedure for Determining Maximum Sustainable Power Generated by Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 1062–1068. [Google Scholar] [CrossRef]
- Boon, N.; De Maeyer, K.; Höfte, M.; Rabaey, K.; Verstraete, W. Use of Pseudomonas species producing phenazine-based metabolites in the anodes of microbial fuel cells to improve electricity generation. Appl. Microbiol. Biotechnol. 2008, 80, 985–993. [Google Scholar]
- Zhang, M.; Ma, Z.; Zhao, N.; Zhang, K.; Song, H. Increased power generation from cylindrical microbial fuel cell inoculated with P. aeruginosa. Biosens. Bioelectron. 2019, 141, 111394. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Khawdas, W.; Aso, Y.; Ohara, H. Microbial fuel cells using Cellulomonas spp. with cellulose as fuel. J. Biosci. Bioeng. 2017, 123, 358–363. [Google Scholar] [CrossRef]
- Masuda, M.; Freguia, S.; Wang, Y.-F.; Tsujimura, S.; Kano, K. Flavins contained in yeast extract are exploited for anodic electron transfer by Lactococcus lactis. Bioelectrochemistry 2010, 78, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ringeisen, B.R.; Ray, R.; Little, B. A miniature microbial fuel cell operating with an aerobic anode chamber. J. Power Sources 2007, 165, 591–597. [Google Scholar] [CrossRef]
- Cordas, C.M.; Guerra, L.T.; Xavier, C.; Moura, J.J.G. Electroactive biofilms of sulphate reducing bacteria. Electrochim. Acta 2008, 54, 29–34. [Google Scholar] [CrossRef]
- Leung, K.M.; Wanger, G.; El-Naggar, M.Y.; Gorby, Y.; Southam, G.; Lau, W.M.; Yang, J. Shewanella oneidensis MR-1 bacterial nanowires exhibit p-type, tunable electronic behavior. Nano Lett. 2013, 13, 2407–2411. [Google Scholar] [CrossRef]
- Keller, K.L.; Rapp-Giles, B.J.; Semkiw, E.S.; Porat, I.; Brown, S.D.; Wall, J.D. New model for electron flow for sulfate reduction in Desulfovibrio alaskensis G20. Appl. Environ. Microbiol. 2014, 80, 855–868. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Li, C.M.; Bao, S.-J.; Lu, Z.; Hong, Y. Direct electrochemistry and electrocatalytic mechanism of evolved Escherichia coli cells in microbial fuel cells. Chem. Comm. 2008, 11, 1290–1292. [Google Scholar] [CrossRef]
- Marshall, C.W.; May, H.D. Electrochemical evidence of direct electrode reduction by a thermophilic Gram-positive bacterium, Thermincola ferriacetica. Energy. Environ. Sci. 2009, 2, 699–705. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Harnisch, F.; Kuhlicke, U.; Neu, T.R.; Schröder, U. Electron transfer and biofilm formation of Shewanella putrefaciens as function of anode potential. Bioelectrochemistry 2013, 93, 23–29. [Google Scholar] [CrossRef]
- Thrash, J.C.; Van Trump, J.I.; Weber, K.A.; Miller, E.; Achenbach, L.A.; Coates, J.D. Electrochemical stimulation of microbial perchlorate reduction. Environ. Sci. Technol. 2007, 41, 1740–1746. [Google Scholar] [CrossRef] [Green Version]
- Kalathil, S.; Pant, D. Nanotechnology to rescue bacterial bidirectional extracellular electron transfer in bioelectrochemical systems. RSC Adv. 2016, 6, 30582–30597. [Google Scholar] [CrossRef] [Green Version]
- Daniel, D.K.; Mankidy, B.D.; Ambarish, K.; Manogari, R. Construction and operation of a microbial fuel cell for electricity generation from wastewater. Int. J. Hydrogen Energy. 2009, 34, 7555–7560. [Google Scholar] [CrossRef]
- Zhi-Dan, L.I.U.; Jing, L.; Zhu-Wei, D.U.; Li, H.-R. Construction of sugar-based microbial fuel cells by dissimilatory metal reduction bacteria. Chin. J. Biotechnol. 2006, 22, 131–137. [Google Scholar]
- Liu, L.; Li, F.-b.; Feng, C.-h.; Li, X.-Z. Microbial fuel cell with an azo-dye-feeding cathode. Appl. Microbiol. Biotechnol. 2009, 85, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.H.A.; Kim, Y.S.; Oh, S.-E. Power generation from cellulose using mixed and pure cultures of cellulose-degrading bacteria in a microbial fuel cell. Enzyme Microb. Technol. 2012, 51, 269–273. [Google Scholar] [CrossRef]
- De Vet, S.J.; Rutgers, R. From waste to energy: First experimental bacterial fuel cells onboard the international space station. Microgravity Sci. Technol. 2007, 19, 225–229. [Google Scholar] [CrossRef]
- Zheng, X.; Nirmalakhandan, N. Cattle wastes as substrates for bioelectricity production via microbial fuel cells. Biotechnol. Lett. 2010, 32, 1809–1814. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Anam, M.; Yousaf, S.; Maleeha, S.; Bangash, Z. Characterization of the electric current generation potential of the pseudomonas aeruginosa using glucose, fructose, and sucrose in double chamber microbial fuel cell. Iran. J. Biotechnol. 2017, 15, 216. [Google Scholar] [CrossRef] [Green Version]
- Khawdas, W.; Watanabe, K.; Karatani, H.; Aso, Y.; Tanaka, T.; Ohara, H. Direct electron transfer of Cellulomonas fimi and microbial fuel cells fueled by cellulose. J. Biosci. Bioeng. 2019, 128, 593–598. [Google Scholar] [CrossRef]
- Powell, E.E.; Mapiour, M.L.; Evitts, R.W.; Hill, G.A. Growth kinetics of Chlorella vulgaris and its use as a cathodic half cell. Bioresour. Technol. 2009, 100, 269–274. [Google Scholar] [CrossRef]
- Cournet, A.; Délia, M.-L.; Bergel, A.; Roques, C.; Bergé, M. Electrochemical reduction of oxygen catalyzed by a wide range of bacteria including Gram-positive. Electrochem. Commun. 2010, 12, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Raghavulu, S.V.; Goud, R.K.; Sarma, P.N.; Mohan, S.V. Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: Influence of redox condition and substrate load. Bioresour. Technol. 2011, 102, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Erable, B.; Vandecandelaere, I.; Faimali, M.; Delia, M.-L.; Etcheverry, L.; Vandamme, P.; Bergel, A. Marine aerobic biofilm as biocathode catalyst. Bioelectrochemistry 2010, 78, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.A.; Lu, Y.; Yang, X.; Shi, X. Carbon and Steel Surfaces Modified by Leptothrix discophora SP-6: Characterization and Implications. Environ. Sci. Technol. 2007, 41, 7987–7996. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Hu, Z.H.; Zhang, L.L.; Yu, X.; Chen, J.M. A novel dichloromethane-degrading Lysinibacillus sphaericus strain wh22 and its degradative plasmid. Appl. Mcrobiol. Biotechnol. 2009, 82, 731–740. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H. New exoelectrogen Citrobacter sp. SX-1 isolated from a microbial fuel cell. J. App. Microbiol. 2011, 111, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Inglesby, A.E.; Beatty, D.A.; Fisher, A.C. Rhodopseudomonas palustris purple bacteria fed Arthrospira maxima cyanobacteria: Demonstration of application in microbial fuel cells. RSC. Adv. 2012, 2, 4829–4838. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.-W.; Li, W.-W.; Sheng, G.-P.; Zang, G.-L.; Cheng, Y.-Y.; Shen, N.; Yang, Y.-P.; Yu, H.-Q. A white-rot fungus is used as a biocathode to improve electricity production of a microbial fuel cell. Appl. Energy 2012, 98, 594–596. [Google Scholar] [CrossRef]
- Huang, H.; Chai, C.; Yang, S.; Jiang, W.; Gu, Y. Phage serine integrase-mediated genome engineering for efficient expression of chemical biosynthetic pathway in gas-fermenting Clostridium ljungdahlii. Metab. Eng. 2019, 52, 293–302. [Google Scholar] [CrossRef]
- Klask, C.-M.; Kliem-Kuster, N.; Molitor, B.; Angenent, L.T. Nitrate Feed Improves Growth and Ethanol Production of Clostridium ljungdahlii with CO2 and H2, but Results in Stochastic Inhibition Events. Front. Microbiol. 2019. [Google Scholar] [CrossRef]
- Mateos, R.; Escapa, A.; Vanbroekhoven, K.; Patil, S.A.; Moran, A.; Pant, D. Microbial electrochemical technologies for CO2 and its derived products valorization. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 777–796. [Google Scholar]
- Kato, S.; Yumoto, I.; Kamagata, Y. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl. Environ. Microbiol. 2015, 81, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, K.V.; Swathi, K.; Hemalatha, M.; Mohan, S.V. Bioelectrocatalyst in Microbial Electrochemical Systems and Extracellular Electron Transport. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 117–141. [Google Scholar]
- Reimers, C.E.; Tender, L.M.; Fertig, S.; Wang, W. Harvesting Energy from the Marine Sediment−Water Interface. Environ. Sci. Technol. 2001, 35, 192–195. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Lee, J.-W.; Kim, K.-Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Borole, A.P.; O’Neill, H.; Tsouris, C.; Cesar, S. A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum. Biotechnol. Lett. 2008, 30, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.H.A.; Zohri, A.A.; Kassim, R.M.F. Electricity generation from sugarcane molasses using microbial fuel cell technologies. Energy 2019, 178, 538–543. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, T.; Lim, B.; Choi, C.; Park, J. Microbial community structures differentiated in a single-chamber air-cathode microbial fuel cell fueled with rice straw hydrolysate. Biotechnol. Biofuels 2014, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.H.A.; Gad El-Rab, S.M.F.; Rahimnejad, M.; Ghasemi, M.; Joo, J.-H.; Sik-Ok, Y.; Kim, I.S.; Oh, S.-E. Electricity generation from rice straw using a microbial fuel cell. Int. J. Hydrogen Energy 2014, 39, 9490–9496. [Google Scholar] [CrossRef]
- Rezaei, F.; Xing, D.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B. E Simultaneous Cellulose Degradation and Electricity Production by Enterobacter cloacae in a Microbial Fuel Cell. Appl. Environ. Microbiol. 2009, 75, 3673. [Google Scholar] [CrossRef] [Green Version]
- Manohar, A.K.; Mansfeld, F. The internal resistance of a microbial fuel cell and its dependence on cell design and operating conditions. Electrochim. Acta 2009, 54, 1664–1670. [Google Scholar] [CrossRef]
- Jung, S.; Regan, J.M. Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl. Microbiol. Biotechnol. 2007, 77, 393–402. [Google Scholar] [CrossRef]
- Zou, Y.; Xiang, C.; Yang, L.; Sun, L.-X.; Xu, F.; Cao, Z. A mediatorless microbial fuel cell using polypyrrole coated carbon nanotubes composite as anode material. Int. J. Hydrogen Energy 2008, 33, 4856–4862. [Google Scholar] [CrossRef]
- Mohan, Y.; Manoj Muthu Kumar, S.; Das, D. Electricity generation using microbial fuel cells. Int. J. Hydrogen Energy 2008, 33, 423–426. [Google Scholar] [CrossRef]
- Ren, Z.; Steinberg, L.M.; Regan, J.M. Electricity production and microbial biofilm characterization in cellulose-fed microbial fuel cells. Water. Sci. Technol. 2008, 58, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.H.A.; Van Ginkel, S.W.; Kim, S.-M.; Yoon, S.-H.; Joo, J.-H.; Shin, B.-S.; Jeon, B.-H.; Bae, W.; Oh, S.-E. Isolation and characterization of Acidithiobacillus caldus from a sulfur-oxidizing bacterial biosensor and its role in detection of toxic chemicals. J. Microbiol. Methods 2010, 82, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-B.; Yong, X.-Y.; Chen, Y.-L.; Liao, Z.-H.; Si, R.-W.; Zhou, J.; Wang, S.-Y.; Yong, Y.-C.; OuYang, P.-K.; Zheng, T. Enhanced bioelectricity generation by improving pyocyanin production and membrane permeability through sophorolipid addition in Pseudomonas aeruginosa-inoculated microbial fuel cells. Bioresour. Technol. 2014, 167, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Jafary, T.; Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Hghparast, F.; Daud, W.R.W. Assessment of bioelectricity production in microbial fuel cells through series and parallel connections. Energy Convers. Manag. 2013, 75, 256–262. [Google Scholar] [CrossRef]
- Akman, D.; Cirik, K.; Ozdemir, S.; Ozkaya, B.; Cinar, O. Bioelectricity generation in continuously-fed microbial fuel cell: Effects of anode electrode material and hydraulic retention time. Bioresour. Technol. 2013, 149, 459–464. [Google Scholar] [CrossRef]
- Mäkinen, A.E.; Lay, C.-H.; Nissilä, M.E.; Puhakka, J.A. Bioelectricity production on xylose with a compost enrichment culture. Int. J. Hydrogen Energy. 2013, 38, 15606–15612. [Google Scholar] [CrossRef]
- Aldrovandi, A.; Marsili, E.; Stante, L.; Paganin, P.; Tabacchioni, S.; Giordano, A. Sustainable power production in a membrane-less and mediator-less synthetic wastewater microbial fuel cell. Bioresour. Technol. 2009, 100, 3252–3260. [Google Scholar] [CrossRef] [Green Version]
- Dumas, C.; Basseguy, R.; Bergel, A. Microbial electrocatalysis with Geobacter sulfurreducens biofilm on stainless steel cathodes. Electrochim. Acta 2008, 53, 2494–2500. [Google Scholar] [CrossRef] [Green Version]
- Catal, T.; Li, K.; Bermek, H.; Liu, H. Electricity production from twelve monosaccharides using microbial fuel cells. J. Power Sources 2008, 175, 196–200. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Christy, A.D.; Dehority, B.A.; Morrison, M.; Yu, Z.; Tuovinen, O.H. Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol. Bioeng. 2007, 97, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Jung, S.H.; Regan, J.M.; Logan, B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 2007, 98, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Kargi, F.; Eker, S. Electricity generation with simultaneous wastewater treatment by a microbial fuel cell (MFC) with Cu and Cu–Au electrodes. J. Chem. Technol. Biotechnol. 2007, 82, 658–662. [Google Scholar] [CrossRef]
- Logan, B.E.; Murano, C.; Scott, K.; Gray, N.D.; Head, I.M. Electricity generation from cysteine in a microbial fuel cell. Water Res. 2005, 39, 942–952. [Google Scholar] [CrossRef]
- Niessen, J.; Schröder, U.; Scholz, F. Exploiting complex carbohydrates for microbial electricity generation–a bacterial fuel cell operating on starch. Electrochem. Commun. 2004, 6, 955–958. [Google Scholar] [CrossRef]
- Kalleary, S.; Abbas, F.M.; Ganesan, A.; Meenatchisundaram, S.; Srinivasan, B.; Packirisamy, A.S.B.; krishnan Kesavan, R.; Muthusamy, S. Biodegradation and bioelectricity generation by microbial desalination cell. Int. Biodeterior. Biodegrad. 2014, 92, 20–25. [Google Scholar] [CrossRef]
- Jayashree, C.; Arulazhagan, P.; Kumar, S.A.; Kaliappan, S.; Yeom, I.T.; Banu, J.R. Bioelectricity generation from coconut husk retting wastewater in fed batch operating microbial fuel cell by phenol degrading microorganism. Biomass. Bioenerg. 2014, 69, 249–254. [Google Scholar] [CrossRef]
- Nimje, V.R.; Chen, C.-Y.; Chen, H.-R.; Chen, C.-C.; Huang, Y.M.; Tseng, M.-J.; Cheng, K.-C.; Chang, Y.-F. Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis. Bioresour. Technol. 2012, 104, 315–323. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kasai, T.; Nakagawa, G.; Yamamuro, A.; Abe, T.; Watanabe, K. Comparative metagenomics of anode-associated microbiomes developed in rice paddy-field microbial fuel cells. PLoS ONE 2013, 8, e77443. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Song, H.-S.; Park, Y.-L.; Han, Y.-H.; Park, J.-Y.; Kim, Y.-G. Chitin biomass powered microbial fuel cell for electricity production using halophilic Bacillus circulans BBL03 isolated from sea salt harvesting area. Bioelectrochemistry 2019, 130, 107329. [Google Scholar] [CrossRef] [PubMed]
- Pushkar, P.; Mungray, A.K. Exploring the use of 3 dimensional low-cost sugar-urea carbon foam electrode in the benthic microbial fuel cell. Renew. Energy 2020, 147, 2032–2042. [Google Scholar] [CrossRef]
- Juan, J.G.; Keegan, G.C.; Marcus, O.G.; Sage, E.R.; Peter, R.G.; Michael, A.C. Benthic microbial fuel cells: Long-term power sources for wireless marine sensor networks. In Proceedings of the SPIE 7666, Sensors, and Command, Control, Communications, and Intelligence (C3I) Technologies for Homeland Security and Homeland Defense IX, Orlando, FL, USA, 5 May 2010. [Google Scholar] [CrossRef]




| Microorganisms | External Mediator | Power Density (mW m−2) | Configurations | Type of Electrons Transfer Mechanisms | References |
|---|---|---|---|---|---|
| Exoelectrogens microorganisms | |||||
| Shewanella oneidensis strain 14063 | 1–amino–2–Napthol | >40 | Single chamber | Direct transfer | [76] |
| Shewanella oneidensis | Anthraquinone–2,6–disulfonate (AQDS) | 24 | Double chamber | Direct transfer | [77] |
| Klebsiella pneumoniae | HNQ as mediator biomineralized manganese as electron acceptor | _ | _ | Direct transfer | [78] |
| Pseudomonas species | phenazine–1–carboxamide | _ | _ | Indirect transfer | [79] |
| Pseudomonas aeruginosa | phenazine compounds | 3322 ± 38 | Single chamber | Direct transfer | [80] |
| Cellulomonas fimi | anthraquinone–2,6–disulfonate | 38.7 | Double chamber | Direct transfer | [81] |
| Lactococcus lactis | Riboflavin, flavins | _ | Double chamber | Direct transfer | [82] |
| Geobacter sulfurreducens | c–Cytochrome z, type IV pili | 3147 | Double chamber | Direct transfer | [83] |
| Shewanella oneidensis DsP10 | Anthraquinone–2,6–disulfonate (AQDS) | 5000 | Double chamber | Direct transfer | [77] |
| Rhodopseudomonas palustris DX-1 | c–Type cytochromes | 2720 | Single chamber | Indirect transfer | [49] |
| Desulfovibrio desulfuricans ATTC | c–Type cytochromes | 1580 | Single chamber | Indirect transfer | [84] |
| Geobacter metallireducens | c–Type cytochromes, OmcE and OmcB | 450 | Single chamber | Indirect transfer | [85] |
| Desulfuromonas acetoxidans | c–Type cytochromes | 2000 | _ | Indirect transfer | [13] |
| Klebsiella pneumonia | 2,6–Di–tert–butyl–p–benzoquinone | 199 | _ | _ | [86] |
| Desulfovibrio alaskensis | Transmembrane complexes, tetraheme cytochrome C3 | _ | _ | _ | [87] |
| Pseudomonas aeruginosa | Phenazine–1–carboxamide, pyocyanin | 4300 | _ | _ | [88] |
| Thermincola ferriacetica | Anthraquinone–2,6–disulfonate | 12,000 | Single chamber | _ | [89] |
| Shewanella putrefaciens | c–Type cytochromes including OmcA, MtrC, FAD transporter | 492 | Double chamber | Indirect transfer | [90] |
| Dechlorospirillum anomalous strain WD | Anthraquinone–2,6–disulfonate hydrogen | 30 | _ | Direct transfer | [91] |
| Geobacter lovleyi | Methyl viologen | 480 | _ | Indirect transfer | [92] |
| Chlorella vulgaris | Methyl viologen, methylene blue | 30 | Single chamber | Indirect transfer | [91] |
| Pseudomonas sp. | Methylene blue | 979 | Single chamber | Indirect transfer | [93] |
| Endoelectrogens microorganism | |||||
| Rhodoferax ferrireducens | _ | 158 | Double chamber | Direct transfer | [94] |
| Klebsiela pneumoniae strain L17 | _ | 34.77 | Double chamber | Direct transfer | [95] |
| Nocardiopsis sp. KNU (strain), Streptomyces enissocaesilis KNU (K strains) | _ | 162 145 | Double chamber | Direct transfer | [96] |
| Rhodoferax ferrireducens | _ | _ | Double chamber | Direct transfer | [97] |
| Escherichia coli strain K-12 | _ | 215 | Single chamber | _ | [98] |
| Shewanella oneidensis | _ | _ | Single chamber | _ | [99] |
| Pseudomonas aeruginosa | _ | 136 ± 87 | Single chamber | _ | [100] |
| Cellulomonas fimi | _ | 0.74 ± 0.07 | Single chamber | Indirect transfer | [101] |
| Leptothrix discophora SP-6 | _ | 70 | _ | Indirect transfer | [102] |
| Acinetobacter calcoaceticus | _ | 110 | _ | Indirect transfer | [50] |
| Escherichia coli | _ | 3390 | _ | [103] | |
| Winogradskyella poriferorum | _ | 40 | _ | Indirect transfer | [104] |
| Pseudomonas fluorescens | _ | 210 | Double chamber | Direct transfer | [105] |
| Citrobacter sp. | _ | 205 | Double chamber | Indirect transfer | [106] |
| Lysinibacillus sphaericus | _ | 850 | Double chamber | Direct transfer | [107] |
| Dechloromonas sp. | _ | 300 | Double chamber | Indirect transfer | [108] |
| Arthrospira maxima | _ | 100 | Double chamber | Direct transfer | [109] |
| Coriolus versicolor | _ | 3200 | Single chamber | Indirect transfer | [110] |
| Waste Substrate | Electircigens | Power Density (mW/m2) | Configurations | Type of Electrons Transfer Mechanisms | References |
|---|---|---|---|---|---|
| Glucose | Acidiphilium cryptum | 12.7 | Single chamber | Direct transfer | [120] |
| Cellulose | Enterobacter cloacae | 5.4 ± 0.3 | Double chamber | Direct transfer | [122] |
| Lactate | Shewanella oneidensis MR-1 | 0.3 × 10−2 | Single chamber | Indirect transfer | [123] |
| Lactate | Geobacter sulfurreducens | 52 ± 4.7 | - | Indirect transfer | [124] |
| Glucose | Escherichia coli | 228 | - | Indirect transfer | [125] |
| Malt extract | Enterobacter cloacae | 9.3 | - | Indirect transfer | [126] |
| Cellulose | G. sulfurreducens and C. cellulolyticum | 83 | Single chamber | Indirect transfer | [127] |
| Wheat straw | Acidithiobacillus caldus | 123 | Single chamber | - | [128] |
| Molasses | B. borstelensis STRI1 | 185.5 | Single chamber | - | [119] |
| Sophorolipid with glucose and PBS | Pseudomonas aeruginosa | 15.29 | Single chamber | - | [129] |
| Glucose, fructose, and sucrose | Saccharomyces cerevisiae | 72.77 | Single chamber | - | [130] |
| Glucose in synthetic wastewater | _ | 1313 | Double chamber | Direct transfer | [131] |
| xylose | Geobacter sulfurreducens Escherichia coli, | 590 | Double chamber | Direct transfer | [132] |
| Synthetic wastewater | α–Proteobacteria, β–Proteobacteria, γ–Proteobacteria | 70 | Double chamber | - | [133] |
| Sodium Fumarate | Geobacter sulfurreducens | _ | Single chamber | - | [134] |
| Glucuronic acid | Rhodococcus sp. and Paracoccus sp. | 2770 | Double chamber | - | [135] |
| Xylose | Clostridium spp. and Comamonas spp. | 1241 | _ | Direct transfer | [136] |
| Acetate | _ | 1430 | _ | [137] | |
| Ethanol | Proteobacterium sp., Azoarcus sp. and Desulfuromonas sp. | 40 | _ | Indirect transfer | [138] |
| Synthetic wastewater with molasses and urea | _ | 2.9 | Single chamber | [139] | |
| Cysteine | Shewanella affinis | 39 | _ | _ | [140] |
| Starch | Clostridium butyricum or Clostridium beijerinckii | _ | _ | _ | [141] |
| Dye-containing wastewater in microbial desalination | Bacillus subtilis, Aeromonas hydrophila subsp. hydrophila | 2.86 | _ | _ | [142] |
| Rice straw | Cellulose-degrading bacteria | 146 | _ | _ | [121] |
| Coconut husk retting | Ochrobactrum sp. | 362 | Double chamber | Indirect transfer | [143] |
| Agriculture wastewater | Shewanella oneidensis | 13 | Double chamber | Indirect transfer | [144] |
| Rice paddy | Geobacteraceae | _ | Double chamber | Indirect transfer | [145] |
| Chitin | Bacillus circulans | 1.742 | Double chamber | Indirect transfer | [146] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, M.F.; Abbas, S.Z.; Mohamad Ibrahim, M.N.; Ismail, N.; Rafatullah, M. Insights into Advancements and Electrons Transfer Mechanisms of Electrogens in Benthic Microbial Fuel Cells. Membranes 2020, 10, 205. https://doi.org/10.3390/membranes10090205
Umar MF, Abbas SZ, Mohamad Ibrahim MN, Ismail N, Rafatullah M. Insights into Advancements and Electrons Transfer Mechanisms of Electrogens in Benthic Microbial Fuel Cells. Membranes. 2020; 10(9):205. https://doi.org/10.3390/membranes10090205
Chicago/Turabian StyleUmar, Mohammad Faisal, Syed Zaghum Abbas, Mohamad Nasir Mohamad Ibrahim, Norli Ismail, and Mohd Rafatullah. 2020. "Insights into Advancements and Electrons Transfer Mechanisms of Electrogens in Benthic Microbial Fuel Cells" Membranes 10, no. 9: 205. https://doi.org/10.3390/membranes10090205