Influences of Combined Organic Fouling and Inorganic Scaling on Flux and Fouling Behaviors in Forward Osmosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Solution (FS), Draw Solution (DS), and Membranes
2.2. FO System Configuration and Filtration Experiments
2.3. Structural and Transport Parameters of the Membrane
2.4. Membrane Surface and Fouling Characterizations
3. Results and Discussion
3.1. FO Membrane Characteristics
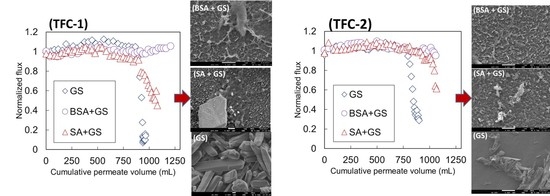
3.2. Flux Profiles during Membrane Fouling
3.3. Analyses of the Membrane Foulants
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chun, Y.; Mulcahy, D.; Zou, L.; Kim, I. A Short Review of Membrane Fouling in Forward Osmosis Processes. Membranes 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- McGovern, R.K.; Lienhard V, J.H. On the potential of forward osmosis to energetically outperform reverse osmosis desalination. J. Membr. Sci. 2014, 469, 245–250. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, J.R. Forward osmosis: A technology platform here to stay. Desalination 2017, 421, 1–2. [Google Scholar] [CrossRef]
- Martinetti, C.R.; Childress, A.E.; Cath, T.Y. High recovery of concentrated RO brines using forward osmosis and membrane distillation. J. Membr. Sci. 2009, 331, 31–39. [Google Scholar] [CrossRef]
- Zhang, J.; Loong, W.L.C.; Chou, S.; Tang, C.; Wang, R.; Fane, A.G. Membrane biofouling and scaling in forward osmosis membrane bioreactor. J. Membr. Sci. 2012, 403–404, 8–14. [Google Scholar] [CrossRef]
- Zaviska, F.; Chun, Y.; Heran, M.; Zou, L. Using FO as pre-treatment of RO for high scaling potential brackish water: Energy and performance optimisation. J. Membr. Sci. 2015, 492, 430–438. [Google Scholar] [CrossRef]
- Fane, A.G. Membranes and the water cycle: Challenges and opportunities. Appl. Water Sci. 2011, 1, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Benecke, J.; Rozova, J.; Ernst, M. Anti-scale effects of select organic macromolecules on gypsum bulk and surface crystallization during reverse osmosis desalination. Sep. Purif. Technol. 2016, 198, 68–78. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, B. Combined fouling of forward osmosis membranes: Synergistic foulant interaction and direct observation of fouling layer formation. J. Membr. Sci. 2012, 407–408, 136–144. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Wicaksana, F.; Tang, C.; Al-Rabiah, A.A.; Al-Zahrani, S.M.; Wang, R. Combined organic–inorganic fouling of forward osmosis hollow fiber membranes. Water Res. 2012, 46, 6329–6338. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Elimelech, M.; Shon, H.K.; Hong, S. Combined organic and colloidal fouling in forward osmosis: Fouling reversibility and the role of applied pressure. J. Membr. Sci. 2014, 460, 206–212. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.-H. Effect of operating conditions on CaSO4 scale formation mechanism in nanofiltration for water softening. Water Res. 2000, 34, 3854–3866. [Google Scholar] [CrossRef]
- Xie, M.; Gray, S.R. Gypsum scaling in forward osmosis: Role of membrane surface chemistry. J. Membr. Sci. 2016, 513, 250–259. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, B. Effects of organic macromolecular conditioning on gypsum scaling of forward osmosis membranes. J. Membr. Sci. 2014, 450, 153–161. [Google Scholar] [CrossRef]
- Lee, E.K.; Chen, V.; Fane, A.G. Natural organic matter (NOM) fouling in low pressure membrane filtration—effect of membranes and operation modes. Desalination 2008, 218, 257–270. [Google Scholar] [CrossRef]
- Le Gouellec, Y.A.; Elimelech, M. Calcium sulfate (gypsum) scaling in nanofiltration of agricultural drainage water. J. Membr. Sci. 2002, 205, 279–291. [Google Scholar] [CrossRef]
- Chun, Y.; Zaviska, F.; Cornelissen, E.; Zou, L. A case study of fouling development and flux reversibility of treating actual lake water by forward osmosis process. Desalination 2015, 357, 55–64. [Google Scholar] [CrossRef]
- Tang, C.Y.; She, Q.; Lay, W.C.L.; Wang, R.; Fane, A.G. Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J. Membr. Sci. 2010, 354, 123–133. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Straub, A.P.; Romero-Vargas Castrillon, S.; Elimelech, M. A method for the simultaneous determination of transport and structural parameters of forward osmosis membranes. J. Membr. Sci. 2013, 444, 523–538. [Google Scholar] [CrossRef]
- Chun, Y.; Qing, L.; Sun, G.; Bilad, M.R.; Fane, A.G.; Chong, T.H. Prototype aquaporin-based forward osmosis membrane: Filtration properties and fouling resistance. Desalination 2018, 445, 75–84. [Google Scholar] [CrossRef]
- Chun, Y.; Mulcahy, D.; Kim, I.S.; Zou, L. The influences of deposited silica nanoparticles on a forward osmosis membrane. Desalin. Water Treat. 2017, 80, 18–26. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Chun, Y.; Mulcahy, D.; Zou, L.; Kim, I.S.; Le-Clech, P. Influence of hydrophobic and electrostatic membrane surface properties on biofouling in a submerged membrane bioreactor under different filtration modes. Desalin. Water Treat. 2016, 57, 26641–26647. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Xia, L.; Andersen, M.F.; Hélix-Nielsen, C.; McCutcheon, J.R. Novel Commercial Aquaporin Flat-Sheet Membrane for Forward Osmosis. Ind. Eng. Chem. Res. 2017, 56, 11919–11925. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Cath, T. A scaling mitigation approach during direct contact membrane distillation. Sep. Purif. Technol. 2011, 80, 315–322. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Chemical and physical aspects of organic fouling of forward osmosis membranes. J. Membr. Sci. 2008, 320, 292–302. [Google Scholar] [CrossRef]
- Lee, S.; Elimelech, M. Relating organic fouling of reverse osmosis membranes to intermolecular adhesion forces. Environ. Sci. Technol. 2006, 40, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Superhydrophilic thin-film composite forward osmosis membranes for organic fouling control: Fouling behavior and antifouling mechanisms. Environ. Sci. Technol. 2012, 46, 11135–11144. [Google Scholar] [CrossRef] [PubMed]
- Hoek, E.M.; Elimelech, M. Cake-enhanced concentration polarization: A new fouling mechanism for salt-rejecting membranes. Environ. Sci. Technol. 2003, 37, 5581–5588. [Google Scholar] [CrossRef] [PubMed]
- Chong, T.H.; Wong, F.S.; Fane, A.G. Implications of critical flux and cake enhanced osmotic pressure (CEOP) on colloidal fouling in reverse osmosis: Experimental observations. J. Membr. Sci. 2008, 314, 101–111. [Google Scholar] [CrossRef]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Miao, R.; Lv, Y.; Wang, X.; Meng, X.; Yang, R.; Zhang, X. Enhanced gypsum scaling by organic fouling layer on nanofiltration membrane: Characteristics and mechanisms. Water Res. 2016, 91, 203–213. [Google Scholar] [CrossRef]
- Chen, S.C.; Wan, C.F.; Chung, T.-S. Enhanced fouling by inorganic and organic foulants on pressure retarded osmosis (PRO) hollow fiber membranes under high pressures. J. Membr. Sci. 2015, 479, 190–203. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Probing the nano- and micro-scales of reverse osmosis membranes—A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements. J. Membr. Sci. 2007, 287, 146–156. [Google Scholar] [CrossRef]
- Chun, Y.; Zaviska, F.; Kim, S.-J.; Mulcahy, D.; Yang, E.; Kim, I.S.; Zou, L. Fouling characteristics and their implications on cleaning of a FO-RO pilot process for treating brackish surface water. Desalination 2016, 394, 91–100. [Google Scholar] [CrossRef]
- Kim, K.J.; Fane, A.G.; Nyström, M.; Pihlajamaki, A. Chemical and electrical characterization of virgin and protein-fouled polycarbonate track-etched membranes by FTIR and streaming-potential measurements. J. Membr. Sci. 1997, 134, 199–208. [Google Scholar] [CrossRef]
- Ramesh, A.; Lee, D.J.; Lai, J.Y. Membrane biofouling by extracellular polymeric substances or soluble microbial products from membrane bioreactor sludge. Appl. Microbiol. Biotechnol. 2007, 74, 699–707. [Google Scholar] [CrossRef]
- Quay, A.N.; Tong, T.; Hashmi, S.M.; Zhou, Y.; Zhao, S.; Elimelech, M. Combined Organic Fouling and Inorganic Scaling in Reverse Osmosis: Role of Protein–Silica Interactions. Environ. Sci. Technol. 2018, 52, 9145–9153. [Google Scholar] [CrossRef] [PubMed]
- Khayet, M.; Cojocaru, C.; Essalhi, M. Artificial neural network modeling and response surface methodology of desalination by reverse osmosis. J. Membr. Sci. 2011, 368, 202–214. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; She, Q.; Fane, A.G.; Field, R.W. Exploring the differences between forward osmosis and reverse osmosis fouling. J. Membr. Sci. 2018, 565, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]




| Membrane ID | m) | Contact Angle (°) | Surface Charge a (mV) | Roughness b (nm) | Pure Water Permeability, A (L m−2 h−1) (LMH)/bar) | Salt Permeability, B (LMH) | A/B (bar−1) | m) | Error (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFC-1 | 115 | 45.5 ± 5.0 (AL) 56.0 ± 2.5 (SL) | −20.9 ± 2.5 | 41.7 ± 1.5 | 3.6 | 1.6 | 2.3 | 600 | 6.65 | 14.3 ± 1.8 | 5.4 ± 1.5 |
| TFC-2 | 87 | 49.1 ± 2.4 (AL) 68.6 ± 1.4 (SL) | −19.3 ± 1.3 | 24.9 ± 2.4 | 11.0 | 5.9 | 1.9 | 220 | 8.87 | 33.3 ± 1.8 | 20.2 ± 1.1 |
| Membrane ID | Type of Foulant | C | O | S | Na | Cl | Ca |
|---|---|---|---|---|---|---|---|
| TFC-1 | Pristine | 85.70 ± 0.10 | 11.93 ± 0.11 | 2.37 ± 0.02 | - * | - | - |
| GS | 9.77 ± 0.26 | 65.98 ± 0.25 | 12.04 ± 0.02 | 0.38 ± 0.02 | 0.35 ± 0.01 | 11.48 ± 0.02 | |
| BSA + GS | 76.30 ± 0.16 | 11.21 ± 0.44 | 2.01 ± 0.25 | 4.79 ± 0.28 | 4.98 ± 0.08 | 0.72 ± 0.18 | |
| SA + GS | 63.53 ± 0.13 | 12.57 ± 0.07 | 4.39 ± 0.01 | 6.78 ± 0.05 | 11.03 ± 0.06 | 1.69 ± 0.04 | |
| TFC-2 | Pristine | 86.12 ± 0.08 | 11.13 ± 0.06 | 2.75 ± 0.03 | - | - | - |
| GS | 65.79 ± 0.17 | 25.69 ± 0.16 | 4.37 ± 0.02 | 0.82 ± 0.01 | 0.93 ± 0.01 | 2.39 ± 0.02 | |
| BSA + GS | 72.39 ± 0.19 | 12.85 ± 0.07 | 1.93 ± 0.38 | 5.89 ± 0.05 | 6.72 ± 0.16 | 0.22 ± 0.03 | |
| SA + GS | 74.87 ± 0.07 | 18.77 ± 0.05 | 2.91 ±0.16 | 0.92 ±0.05 | 1.20 ± 0.14 | 1.33 ± 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, Y.; Jeong, K.; Cho, K.H. Influences of Combined Organic Fouling and Inorganic Scaling on Flux and Fouling Behaviors in Forward Osmosis. Membranes 2020, 10, 115. https://doi.org/10.3390/membranes10060115
Chun Y, Jeong K, Cho KH. Influences of Combined Organic Fouling and Inorganic Scaling on Flux and Fouling Behaviors in Forward Osmosis. Membranes. 2020; 10(6):115. https://doi.org/10.3390/membranes10060115
Chicago/Turabian StyleChun, Youngpil, Kwanho Jeong, and Kyung Hwa Cho. 2020. "Influences of Combined Organic Fouling and Inorganic Scaling on Flux and Fouling Behaviors in Forward Osmosis" Membranes 10, no. 6: 115. https://doi.org/10.3390/membranes10060115