Zinc Chloride Enhances the Antioxidant Status, Improving the Functional and Structural Organic Disturbances in Streptozotocin-Induced Diabetes in Rats
Abstract
:1. Introduction
2. Material and Method
2.1. Substances
2.2. Animals
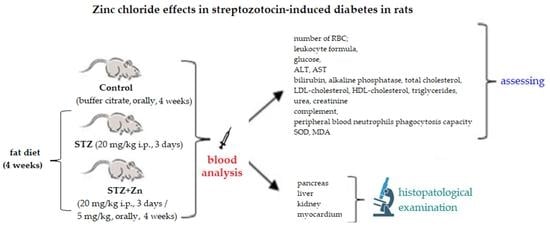
2.3. The Experimental Protocol
2.3.1. The Experimental Model of STZ-Induced Diabetes in Rats
- Group 1 (coded Control): buffer citrate 0.1 mL/100 g body;
- Group 2 (coded STZ): 20 mg/kg body STZ and fat diet (10 g cholesterol/100 g diet), for 4 weeks;
- Group 3 (coded STZ+Zn): 20 mg/kg body STZ + 5 mg/kg body Zn chloride and fat diet (10 g cholesterol/100 g diet), for 4 weeks.
2.3.2. Laboratory Investigations
2.3.3. Histologic Examination
2.4. Ethical Aspects of the Research
2.5. Statistical Analysis of Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ke, C.; Narayan, K.M.V.; Chan, J.C.N.; JHa, P.; Shan, B.R. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat. Rev. Endocrinol. 2022, 18, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab. Syndr. Obes. 2021, 14, 3567–3602. [Google Scholar] [CrossRef] [PubMed]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomic, D.; Shaw, J.E.; Magliano, D. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Liu, R.; Li, L.; Shao, C.; Cai, H.; Wang, Z. The impact of diabetes on vascular disease: Progress from the perspective of epidemics and treatments. J. Diabetes Res. 2022, 2022, 1531289. [Google Scholar] [CrossRef]
- Alu, S.N.; Los, E.A.; Ford, G.A.; Stone, W.L. Oxidative stress in type 2 diabetes: The case for future pediatric redoxomics studies. Antioxidants 2022, 11, 1336. [Google Scholar] [CrossRef]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Salceda, R. High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Müller retinal cells in vitro. Sci. Rep. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Taslimi, P.; Aslan, H.E.; Demir, Y.; Oztaskin, N.; Maraş, A.; Gulçin, İ.; Beydemir, S.; Goksu, S. Diarylmethanon, bromophenol and diarylmethane compounds: Discovery of potent aldose reductase, α-amylase and α-glycosidase inhibitors as new therapeutic approach in diabetes and functional hyperglycemia. Int. J. Biol. Macromol. 2018, 119, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, L.; Shukla, S.; Crane, J.S. Zinc Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493231/#:~:text=Zinc%20deficiency%20is%20commonly%20seen,can%20be%20acquired%20or%20inherited (accessed on 14 September 2022).
- Hussain, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad MDar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanistic impact of zinc deficiency in human development. Front. Nutr. 2022, 9, 717064. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, J.; Feng, J.; Cao, B. Zinc in cognitive impairment and aging. Biomolecules 2022, 12, 1000. [Google Scholar] [CrossRef]
- Sangeetha, V.J.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Zinc nutrition and human health: Overview and implications. eFood 2022, 3, e17. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc and the Immune System. In Nutrition and Immunity; Springer: Berlin/Heidelberg, Germany, 2019; pp. 127–158. [Google Scholar]
- Amos, A.; Razzaque, M.S. Zinc and its role in vitamin D function. Curr. Res. Physiol. 2022, 5, 203–207. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Z.; Liu, S.; Aluo, Z.; Zhang, L.; Yu, L.; Li, Y.; Song, Z.; Zhou, L. Zinc supplementation alleviates lipid and glucose metabolic disorders induced by a high-fat diet. J. Agric. Food Chem. 2020, 68, 5189–5200. [Google Scholar] [CrossRef] [PubMed]
- Pompano, L.M.; Boy, E. Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 141–160. [Google Scholar] [CrossRef]
- Atari-Hajipirloo, S.; Valizadeh, N.; Khadem-Ansari, M.; Rasmi, Y.; Kheradmand, F. Altered concentrations of copper, zinc, and iron are associated with increased levels of glycated hemoglobin in patients with type 2 diabetes mellitus and their first-degree relatives. Int. J. Endocrinol. Metab. 2016, 14, e33273. [Google Scholar] [CrossRef] [Green Version]
- Thapa, B.; Suh, E.H.; Parrott, D.; Khalighinejad, P.; Sharma, G.; Chirayil, S.; Sherry, A.D. Imaging β-cell function using a zinc-responsive MRI contrast agent may identify first responder islets. Front. Endocrinol. 2022, 12, 809867. [Google Scholar] [CrossRef]
- Merriman, C.; Fu, D. Down-regulation of the islet-specific zinc transporter-8 (ZnT8) protects human insulinoma cells against inflammatory stress. J. Biol. Chem. 2019, 294, 16992–17006. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Prev. Nutr. Food Sci. 2017, 22, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Roney, N. Toxicological Profile for Zinc; Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine: Atlanta, GA, USA, 2005.
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Khan, M.; Sheikh, T.M.M.; Mumtaz, M.Z.; Chohan, T.A.; Shamim, S.; Liu, Y. Zinc essentiality, toxicity, and its bacterial bioremediation: A comprehensive insight. Front. Microbiol. 2022, 13, 900740. [Google Scholar] [CrossRef]
- Mustafa, S.; AlSharif, M. Copper (Cu) an essential redox-active transition metal in living system—A review article. AJAC 2018, 9, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Komarnicka, U.K.; Lesiów, M.K.; Witwicki, M.; Bie’nko, A. The bright and dark sides of reactive oxygen species generated by copper–peptide complexes. Separations 2022, 9, 73. [Google Scholar] [CrossRef]
- Grădinaru, D.; Margină, D.; Ungurianu, A.; Nițulescu, G.; Pena, C.M.; Ionescu-Tîrgoviște, C.; Dănciulescu Miulescu, R. Zinc status, insulin resistance and glycoxidative stress in elderly subjects with type 2 diabetes mellitus. Exp. Ther. Med. 2021, 22, 1393. [Google Scholar] [CrossRef]
- Martins, M.P.S.C.; da Silva Santos Oliveira, A.S.; de Carvalho e Martins, M.C.; de Carvalho, V.B.L.; Rodrigues, L.A.R.L.; Arcanjo, D.D.R.; dos Santos, M.A.P.; Machado, J.S.R.; de Moura Rocha, M. Effects of zinc supplementation on glycemic control and oxidative stress in experimental diabetes: A systematic review. Clin. Nutr. ESPEN 2022, 51, 28–36. [Google Scholar] [CrossRef]
- Farooq, D.M.; Alamri, A.F.; Alwhahabi, B.K.; Metwally, A.M.; Kareem, K.A. The status of zinc in type 2 diabetic patients and its association with glycemic control. J. Fam. Community Med. 2020, 27, 29–36. [Google Scholar] [CrossRef]
- MacKenzie, S.; Bergdahl, A. Zinc homeostasis in diabetes mellitus and vascular complications. Biomedicines 2022, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Nazem, M.R.; Asadi, M.; Adelipour, M.; Jabbari, N.; Allameh, A. Zinc supplementation ameliorates type 2 diabetes markers through the enhancement of total antioxidant capacity in overweight patients. Postgrad. Med. J. 2022, 2021-140878. [Google Scholar] [CrossRef]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.; de Oliveira, A.R.; Marreiro Ddo, N. Antioxidant role of zinc in diabetes mellitus. World J. Diabetes 2015, 6, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, N.; Naderi, M.; Noroozi, A.; Ghasemi, M.; Zamani, E.; Shaki, F. Zinc deficiency and oxidative stress involved in valproic acid induced hepatotoxicity: Protection by zinc and selenium supplementation. Biol. Trace Elem. Res. 2017, 179, 102–109. [Google Scholar] [CrossRef]
- Seet, R.C.; Lee, C.Y.; Lim, E.C.; Quek, A.M.; Huang, H.; Huang, S.H.; Looi, W.F.; Long, L.H.; Halliwell, B. Oral zinc supplementation does not improve oxidative stress or vascular function in patients with type 2 diabetes with normal zinc levels. Atherosclerosis 2011, 219, 231–239. [Google Scholar] [CrossRef]
- Yu, J.; Lee, S.H.; Kim, M.K. Recent updates to clinical practice guidelines for diabetes mellitus. Endocrinol. Metab. 2022, 37, 26–37. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Dabirinejhad, H.; Dayer, M.R.; Mohammadi, T. Effect of zinc supplementation on some biochemical and hematological parameters in alloxan induced diabetic rats. JABS 2022, 12, 94–105. [Google Scholar] [CrossRef]
- Yaghmaei, P.; Esfahani-Nejad, H.; Ahmadi, R.; Hayati-Roodbari, N.; Ebrahim-Habibi, A. Maternal zinc intake of Wistar rats has a protective effect in the alloxan-induced diabetic offspring. J. Physiol. Biochem. 2013, 69, 35–43. [Google Scholar] [CrossRef]
- Barman, S.; Srinivasan, K. Zinc supplementation alleviates hyperglycemia and associated metabolic abnormalities in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2016, 94, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Pillai, S.I.; Subramanian, S.P. Synthesis, spectral characterization, and biochemical evaluation of antidiabetic properties of a new zinc-diosmin complex studied in high fat diet fed-low dose streptozotocin induced experimental type 2 diabetes in rats. Biochem. Res. Int. 2015, 2015, 350829. [Google Scholar] [CrossRef] [Green Version]
- Turkyilmaz, I.B.; Bayrak, B.B.; Sacan, O.; Mutlu OAkev, N.; Yanardag, R. Zinc supplementation restores altered biochemical parameters in stomach tissue of STZ diabetic rats. Biol. Trace Elem. Res. 2021, 199, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Ryadinency, R.; Hadisaputro, S.; Rachmawati, B. Effect of zinc supplementation on triglyceride and malondialdehyde levels: Study on diabetic Wistar rats induced with streptozotocin. Med. J. Indones. 2018, 27, 82. [Google Scholar] [CrossRef] [Green Version]
- Barman, S.; Srinivasan, K. Attenuation of oxidative stress and cardioprotective effects of zinc supplementation in experimental diabetic rats. Br. J. Nutr. 2017, 117, 335–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukperoro, J.U.; Offiah, N.; Idris, T.; Awogoke, D. Antioxidant effect of zinc, selenium and their combination on the liver and kidney of alloxan-induced diabetes in rats. Mediterr. J. Nutr. Metab. 2010, 3, 25–30. [Google Scholar] [CrossRef]
- Kim, E.; Sohn, S.; Lee, M.; Jung, J.; Kineman, R.D.; Park, S. Differential responses of the growth hormone axis in two rat models of streptozotocin-induced insulinopenic diabetes. J. Endocrinol. 2006, 188, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Goosens, K.A. Sampling blood from the lateral tail vein of the rat. J. Vis. Exp. 2015, 99, e52766. [Google Scholar] [CrossRef] [Green Version]
- Fischbach, F. Blood studies: Hematology and coagulation; Appendix J: Effects of the most commonly used drugs on frequently ordered laboratory tests. In A Manual of Laboratory and Diagnostic Tests, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; pp. 67–110, 1227–1247. ISBN 978-1-4511-9089-2. [Google Scholar]
- Fischbach, F. Chemistry Studies. In A Manual of Laboratory and Diagnostic Tests, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; pp. 452–455. ISBN 978-1-4511-9089-2. [Google Scholar]
- Marques-Garcia, F. Methods for hemolysis interference study in laboratory medicine—A critical review. EJIFCC 2020, 31, 85–97. [Google Scholar]
- Yusof, N.; Yasin, N.M.; Yousuf, R.; Wahab, A.A.; Aziz, S.A. Comparison of neutrophil respiratory oxidative burst activity between flow cytometry using dihydrorhodamine (DHR) 123 and conventional nitroblue tetrazolium test (NBT). BJMS 2022, 21, 626–633. [Google Scholar] [CrossRef]
- Lima, M.C.; Marks, G.; Silva, I.S.; Silva, B.A.; Cônsolo, L.Z.; Nogueira, G.B. Evaluation of oxidative stress in mice subjected to aerobic exercise. Acta Cir. Bras. 2012, 27, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 6 September 2022).
- Knez, M.; Glibetic, M. Zinc as a biomarker of cardiovascular health. Front. Nutr. 2021, 8, 686078. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y. The role of zinc homeostasis in the prevention of diabetes mellitus and cardiovascular diseases. J. Atheroscler. Thromb. 2021, 28, 1109–1122. [Google Scholar] [CrossRef]
- Wang, Z.; Ronsmans, C.; Woolf, B. Triangulating evidence for the causal impact of single-intervention zinc supplement on glycaemic control for type 2 diabetes: Systematic review and meta-analysis of randomised controlled trial and two-sample Mendelian randomisation. Br. J. Nutr. 2022, 10, 1–16. [Google Scholar] [CrossRef]
- Yan, L.-J. The nicotinamide/streptozotocin rodent model of type 2 diabetes: Renal pathophysiology and redox imbalance features. Biomolecules 2022, 12, 1225. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Hübner, C.; Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 2021, 41, 101916. [Google Scholar] [CrossRef]
- Frassinetti, S.; Bronzetti, G.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Liu, Y.; Li, H.; Wang, X.; Wu, W.; Gao, L. Effect and mechanisms of zinc supplementation in protecting against diabetic cardiomyopathy in a rat model of type 2 diabetes. Bosn. J. Basic Med. Sci. 2015, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Visconti, L.; Giuffrida, A.E.; Labbozzetta, V.; Peritore, L.; Lipari, A.; Calabrese, V.; Piccoli, G.B.; Torreggiani, M.; Siligato, R.; et al. Role of zinc in diabetic kidney disease. Nutrients 2022, 14, 1353. [Google Scholar] [CrossRef]
- Yakhchalian, H.; Mohammadian, N.; Hatami, K.; Nosrati, H.; Yousofvand, N. Hematological and serum biochemical analysis of streptozotocin-induced insulin dependent diabetes mellitus in male adult wistar rats. bioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- Hajam, Y.A.; Rai, S.; Ghosh, H.; Basheer, M. Combined administration of exogenous melatonin and insulin ameliorates streptozotocin induced toxic alteration on hematological parameters in diabetic male Wistar rats. Toxicol. Rep. 2020, 7, 353–359. [Google Scholar] [CrossRef]
- Mahmoud, A.M. Hematological alterations in diabetic rats—Role of adipocytokines and effect of citrus flavonoids. EXCLI J. 2013, 12, 647–657. [Google Scholar]
- Nagarajan, S.R.; Paul-Heng, M.; Krycer, J.R.; Fazakerley, D.J.; Sharland, A.F.; Hoy, A.J. Lipid and glucose metabolism in hepatocyte cell lines and primary mouse hepatocytes: A comprehensive resource for in vitro studies of hepatic metabolism. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E578–E589. [Google Scholar] [CrossRef]






| Moment of Evaluation | RBC (mil/μL) | |
|---|---|---|
| Control | Baseline | 8.56 ± 1.27 |
| after 1 week | 8.43 ± 1.65 | |
| after 4 weeks | 8.38 ± 1.22 | |
| STZ | Baseline | 8.64 ± 1.32 |
| after 1 week | 8.48 ± 1.47 | |
| after 4 weeks | 8.35 ± 1.18 | |
| STZ+Zn | Baseline | 8.61 ± 2.15 |
| after 1 week | 8.52 ± 1.25 | |
| after 4 weeks | 8.46 ± 1.30 |
| Moment of Evaluation | Leukocyte Formula Elements (%) | |||||
|---|---|---|---|---|---|---|
| PMN | Ly | E | M | B | ||
| Control | Baseline | 27.4 ± 4.25 | 68.4 ± 7.39 | 0.3 ± 0.01 | 3.7 ± 0.03 | 0.2 ± 0.01 |
| after 1 week | 27.1 ± 4.11 | 68.6 ± 8.42 | 0.5 ± 0.03 | 3.6 ± 0.05 | 0.2 ± 0.01 | |
| after 4 weeks | 27.2 ± 4.67 | 68.9 ± 8.33 | 0.4 ± 0.03 | 3.3 ± 0.05 | 0.2 ± 0.03 | |
| STZ | Baseline | 26.8 ± 3.83 | 69.2 ± 8.27 | 0.4 ± 0.03 | 3.4 ± 0.01 | 0.2 ± 0.01 |
| after 1 week | 27.5 ± 5.17 | 68.5 ± 7.53 | 0.3 ± 0.01 | 3.5 ± 0.03 | 0.2 ± 0.01 | |
| after 4 weeks | 27.3 ± 4.11 | 68.7 ± 8.42 | 0.3 ± 0.03 | 3.5 ± 0.05 | 0.2 ± 0.01 | |
| STZ+Zn | Baseline | 26.7 ± 3.45 | 69.3 ± 8.67 | 0.3 ± 0.03 | 3.5 ± 0.05 | 0.2 ± 0.01 |
| after 1 week | 26.9 ± 4.30 | 68.8 ± 9.22 | 0.4 ± 0.01 | 3.7 ± 0.05 | 0.2 ± 0.01 | |
| after 4 weeks | 27.0 ± 4.55 | 68.9 ± 8.17 | 0.5 ± 0.01 | 3.4 ± 0.03 | 0.2 ± 0.03 | |
| Moment of Evaluation | ALT (U/L) | AST (U/L) | |
|---|---|---|---|
| Control | Baseline | 40.54 ± 4.29 | 91.64 ± 7.27 |
| after 1 week | 42.72 ± 5.55 | 90.87 ± 7.43 | |
| after 4 weeks | 41.36 ± 4.17 | 91.25 ± 6.67 | |
| STZ | Baseline | 40.63 ± 4.83 | 90.45 ± 6.33 |
| after 1 week | 48.24 ± 4.45 | 113.39 ± 7.45 | |
| after 4 weeks | 69.33 ± 5.22 *♦ | 124.63 ± 7.18 *♦ | |
| STZ+Zn | Baseline | 41.48 ± 4.67 | 91.55 ± 6.83 |
| after 1 week | 48.51 ± 5.13 | 100.36 ± 6.33 | |
| after 4 weeks | 45.75 ± 5.05 | 99.48 ± 6.29 |
| Moment of Evaluation | Total Bilirubin (mg/dL) | Alkaline Phosphatase (U/L) | |
|---|---|---|---|
| Control | Baseline | 0.82 ± 0.03 | 121.56 ± 7.22 |
| after 1 week | 0.79 ± 0.01 | 123.25 ± 8.13 | |
| after 4 weeks | 0.80 ± 0.03 | 122.32 ± 7.67 | |
| STZ | Baseline | 0.85 ± 0.07 | 120.63 ± 7.43 |
| after 1 week | 0.77 ± 0.05 | 127.45 ± 6.83 | |
| after 4 weeks | 0.76 ± 0.03 | 138.52 ± 8.33 *♦ | |
| STZ+Zn | Baseline | 0.81 ± 0.01 | 121.39 ± 7.67 |
| after 1 week | 0.78 ± 0.05 | 124.67 ± 7.55 | |
| after 4 weeks | 0.79 ± 0.03 | 123.84 ± 7.27 |
| Moment of Evaluation | Total Cholesterol (mg/dL) | LDL-Cholesterol (mg/dL) | HDL-Cholesterol (mg/dL) | Triglycerides (mg/dL) | |
|---|---|---|---|---|---|
| Control | Baseline | 65.48 ± 5.67 | 23.53 ± 1.11 | 45.44 ± 2.43 | 51.82 ± 4.25 |
| after 1 week | 64.33 ± 5.21 | 21.61 ± 1.43 | 46.62 ± 2.17 | 52.25 ± 4.67 | |
| after 4 weeks | 67.26 ± 6.30 | 24.85 ± 1.05 | 45.73 ± 1.55 | 52.16 ± 5.43 | |
| STZ | Baseline | 64.82 ± 4.13 | 22.48 ± 1.17 | 45.35 ± 2.21 | 52.44 ± 5.21 |
| after 1 week | 78.63 ± 6.45 *♦ | 25.32 ± 1.55 | 35.36 ± 2.67 | 60.59 ± 5.33 | |
| after 4 weeks | 80.54 ± 6.33 *♦ | 29.64 ± 2.13 | 34.28 ± 2.05 *♦ | 63.78 ± 6.17 *♦ | |
| STZ+Zn | Baseline | 66.42 ± 4.55 | 21.71 ± 1.22 | 46.14 ± 2.33 | 51.34 ± 4.83 |
| after 1 week | 74.37 ± 5.05 | 26.12 ± 1.35 | 36.65 ± 1.29 | 56.26 ± 4.79 | |
| after 4 weeks | 75.76 ± 5.83 | 27.24 ± 2.33 | 37.30 ± 1.43 | 54.26 ± 5.55 |
| Moment of Evaluation | Urea (mg/dL) | Creatinine (mg/dL) | |
|---|---|---|---|
| control | Baseline | 40.12 ± 3.33 | 0.13 ± 0.01 |
| after 1 week | 42.05 ± 3.41 | 0.14 ± 0.01 | |
| after 4 weeks | 42.28 ± 13.17 | 0.12 ± 0.03 | |
| STZ | Baseline | 40.26 ± 3.17 | 0.13 ± 0.03 |
| after 1 week | 47.55 ± 4.43 | 0.15 ± 0.01 | |
| after 4 weeks | 59.83 ± 6.13 *♦ | 0.26 ± 0.03 *♦ | |
| STZ+Zn | Baseline | 39.45 ± 3.67 | 0.12 ± 0.01 |
| after 1 week | 43.24 ± 3.55 | 0.14 ± 0.01 | |
| after 4 weeks | 44.58 ± 3.82 | 0.14 ± 0.03 |
| Moment of Evaluation | Complement (UCH50) | NBT (%) | |
|---|---|---|---|
| Control | Baseline | 53.50 ± 4.43 | 13.33 ± 1.55 |
| after 1 week | 54.42 ± 4.17 | 14.17 ± 1.83 | |
| after 4 weeks | 54.38 ± 4.30 | 14.56 ± 2.67 | |
| STZ | Baseline | 53.83 ± 4.29 | 13.52 ± 1.43 |
| after 1 week | 62.94 ± 5.13 | 11.21 ± 1.17 | |
| after 4 weeks | 63.78 ± 5.22 | 11.05 ± 1.29 | |
| STZ+Zn | Baseline | 53.25 ± 3.41 | 14.78 ± 1.22 |
| after 1 week | 55.46 ± 4.88 | 13.24 ± 1.05 | |
| after 4 weeks | 56.74 ± 4.55 | 12.53 ± 1.13 |
| Moment of Evaluation | SOD (U/mg Protein) | MDA (nMol/mg Protein) | |
|---|---|---|---|
| Control | Baseline | 104.48 ± 6.43 | 22.54 ± 1.35 |
| after 1 week | 105.63 ± 6.55 | 23.27 ± 2.11 | |
| after 4 weeks | 106.39 ± 7.27 | 22.65 ± 1.67 | |
| STZ | Baseline | 105.22 ± 6.43 | 22.83 ± 1.43 |
| after 1 week | 98.33 ± 4.51 | 29.48 ± 2.13 | |
| after 4 weeks | 79.85 ± 5.13 *♦ | 32.67 ± 1.25 *♦ | |
| STZ+Zn | Baseline | 105.34 ± 5.83 | 21.25 ± 1.33 |
| after 1 week | 103.62 ± 6.67 | 26.71 ± 2.21 | |
| after 4 weeks | 104.29 ± 6.21 | 25.44 ± 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anton, I.C.; Mititelu-Tartau, L.; Popa, E.G.; Poroch, M.; Poroch, V.; Pelin, A.-M.; Pavel, L.L.; Drochioi, I.C.; Botnariu, G.E. Zinc Chloride Enhances the Antioxidant Status, Improving the Functional and Structural Organic Disturbances in Streptozotocin-Induced Diabetes in Rats. Medicina 2022, 58, 1620. https://doi.org/10.3390/medicina58111620
Anton IC, Mititelu-Tartau L, Popa EG, Poroch M, Poroch V, Pelin A-M, Pavel LL, Drochioi IC, Botnariu GE. Zinc Chloride Enhances the Antioxidant Status, Improving the Functional and Structural Organic Disturbances in Streptozotocin-Induced Diabetes in Rats. Medicina. 2022; 58(11):1620. https://doi.org/10.3390/medicina58111620
Chicago/Turabian StyleAnton, Irina Claudia, Liliana Mititelu-Tartau, Eliza Gratiela Popa, Mihaela Poroch, Vladimir Poroch, Ana-Maria Pelin, Liliana Lacramioara Pavel, Ilie Cristian Drochioi, and Gina Eosefina Botnariu. 2022. "Zinc Chloride Enhances the Antioxidant Status, Improving the Functional and Structural Organic Disturbances in Streptozotocin-Induced Diabetes in Rats" Medicina 58, no. 11: 1620. https://doi.org/10.3390/medicina58111620