Molecular Basis of Cancer Pain Management: An Updated Review
Abstract
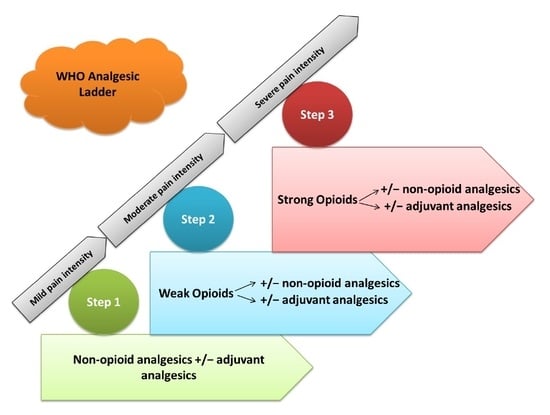
:1. Introduction
2. Cancer and Analgesics
3. Pain Medication, Opioid Analgesics and Non-Steroidal Anti-Inflammatory Drugs (NSAID) in Cancer Pain Relief
3.1. Morphine
3.2. Codeine
3.3. Hydrocodone
3.4. Hydromorphone
3.5. Fentanyl
- Fentanyl is a highly lipophilic opioid and is used for relieving cancer pain in transdermal and transmucosal immediate-release formulations [18]. It is metabolized primarily by CYP3A4/5 to the inactive metabolite, norfentanyl [3]. A118G polymorphisms of OPRM1 were present in various Asian cohorts’ post-surgery and revealed lower fentanyl requirements in A118G-homozygous individuals [26]. The use of fentanyl for children above the age of 2 years has been approved by the FDA and it is one of the most commonly used analgesics amongst pediatric patients [9]. Comparisons made between morphine and transdermal fentanyl have shown an equal analgesic efficacy [8]. Fentanyl can be administered by continuous intravenous or subcutaneous infusion [18]. All the studies found transdermal fentanyl to be cost-effective against oral sustained release morphine with incremental cost-effectiveness ratios of £17,798, £14,487 and £1406 per quality adjusted life years in the studies by Neighbors et al. [27], Radbruch et al. [28] and Greiner et al. [29], respectively for cancer and non-cancer patients with moderate to severe chronic pain [30]. In one study of 60 adult patients with cancer receiving transdermal fentanyl, showed that polymorphisms in the gene ABCB1 could lead to significant changes in fentanyl plasma concentrations, with the ABCB1 1236TT variant being associated with a lower need for rescue medication. To date there have been no statistically significant findings for fentanyl-related adverse effects, in the previous study or current body of literature [31,32].
3.6. Sufentanyl
3.7. Methadone
3.8. Levorphanol
3.9. Buprenorphine
3.10. Oxycodone
3.11. Tramadol
3.12. Tapentadol
3.13. Non-Steroidal Anti-Inflammatory Drugs (NSAID)
3.14. Paracetamol (Acetaminophen)
3.15. Nefopam
3.16. Metamizole (Dipyrone)
4. Adjuvant Analgesics in Cancer
4.1. Antidepressants
4.2. Corticosteroids
4.3. Anticonvulsants (Gabapentinoids)
4.4. Anesthetics
4.5. Ketamine
4.6. Neuroleptics
4.7. Bisphosphonates
4.8. Cannabinoids
4.9. Dexmedetomidine
5. Enzymes Involved in Drug Metabolism
5.1. CYP2D6
5.2. CYP2C9
5.3. Opioid Receptors
5.4. Adenosine Triphosphate-Binding Cassette, Sub-Family B, Member 1 (ABCB1)
5.5. Catechol-O-Methyltransferase (COMT)
5.6. Uridine Diphosphate Glucuronosyltransferases (UGTs)
5.7. Melanocortin-1 Receptor Gene
6. Conclusions and Future Perspective
7. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nersesyan, H.; Slavin, K.V. Current aproach to cancer pain management: Availability and implications of different treatment options. Ther. Clin. Risk Manag. 2007, 3, 381–400. [Google Scholar] [PubMed]
- Kurita, G.P.; Sjøgren, P.; Klepstad, P.; Mercadante, S. Interventional Techniques to Management of Cancer-Related Pain: Clinical and Critical Aspects. Cancers 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.C.; Donovan, K.A.; McLeod, H.L. Clinical Implications of Opioid Pharmacogenomics in Patients with Cancer. Cancer Control J. Moffitt Cancer Cent. 2015, 22, 426–432. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer Pain Relief and Palliative Care: Report of a WHO Expert Committee [Meeting Held in Geneva from 3 to 10 July 1989]; World Health Organization: Geneva, Switzerland, 1990; ISBN 978-92-4-120804-8. [Google Scholar]
- Hanks, G.W.; Conno, F.; Cherny, N.; Hanna, M.; Kalso, E.; McQuay, H.J.; Mercadante, S.; Meynadier, J.; Poulain, P.; Ripamonti, C.; et al. Morphine and alternative opioids in cancer pain: The EAPC recommendations. Br. J. Cancer 2001, 84, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W. Progress in pharmacological pain treatment with opioid analgesics. Contemp. Oncol. Onkol. 2009, 13, 66–73. [Google Scholar]
- Eidelman, A.; White, T.; Swarm, R.A. Interventional therapies for cancer pain management: Important adjuvants to systemic analgesics. J. Natl. Compr. Cancer Netw. JNCCN 2007, 5, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Prommer, E.E. Pharmacological Management of Cancer-Related Pain. Cancer Control J. Moffitt Cancer Cent. 2015, 22, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Constance, J.E.; Campbell, S.C.; Somani, A.A.; Yellepeddi, V.; Owens, K.H.; Sherwin, C.M.T. Pharmacokinetics, pharmacodynamics and pharmacogenetics associated with nonsteroidal anti-inflammatory drugs and opioids in pediatric cancer patients. Expert Opin. Drug Metab. Toxicol. 2017, 13, 715–724. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Gharibo, C.; Ho, K.-Y. Treatment Considerations for Cancer Pain: A Global Perspective. Pain Pract. Off. J. World Inst. Pain 2015, 15, 778–792. [Google Scholar] [CrossRef]
- Juneja, R. Opioids and cancer recurrence. Curr. Opin. Support. Palliat. Care 2014, 8, 91–101. [Google Scholar] [CrossRef]
- Bruera, E.; Paice, J.A. Cancer pain management: Safe and effective use of opioids. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e593–e599. [Google Scholar] [CrossRef] [PubMed]
- Kurita, G.P.; Sjøgren, P. Pain management in cancer survivorship. Acta Oncol. Stockh. Swed. 2015, 54, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Bayonas, A.; Jiménez-Fonseca, P.; Castañón, E.; Ramchandani-Vaswani, A.; Sánchez-Bayona, R.; Custodio, A.; Calvo-Temprano, D.; Virizuela, J.A. Chronic opioid therapy in long-term cancer survivors. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2017, 19, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.J.S.; Stevens, B.J.; McGrath, P.J. Pain in Neonates and Infants; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-52061-6. [Google Scholar]
- Jimenez, N.; Galinkin, J.L. Personalizing pediatric pain medicine: Using population-specific pharmacogenetics, genomics, and other -omics approaches to predict response. Anesth. Analg. 2015, 121, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; van den Anker, J.N. How to use drugs for pain management: From pharmacokinetics to pharmacogenomics. Eur. J. Pain Suppl. 2008, 2, 25–30. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Ahmed, E. Principles of opioid use in cancer pain. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Landau, R. Pharmacogenomic Considerations in Opioid Analgesia. Available online: https://www.dovepress.com/pharmacogenomic-considerations-in-opioid-analgesia-peer-reviewed-article-PGPM (accessed on 12 November 2016).
- Moradkhani, M.R.; Karimi, A. Role of Drug Anesthesia and Cancer. Drug Res. 2018, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.-M.; Wong, C.-S.; Wu, J.-Y.; Chen, Y.-T. Pharmacogenomics for personalized pain medicine. Acta Anaesthesiol. Taiwan 2016, 54, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Drendel, A. Pharmacogenomics of Analgesic Agents. Clin. Pediatr. Emerg. Med. 2007, 8, 262–267. [Google Scholar] [CrossRef]
- Dean, M. Opioids in renal failure and dialysis patients. J. Pain Symptom Manag. 2004, 28, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Manworren, R.C.B.; Jeffries, L.; Pantaleao, A.; Seip, R.; Zempsky, W.T.; Ruaño, G. Pharmacogenetic Testing for Analgesic Adverse Effects: Pediatric Case Series. Clin. J. Pain 2016, 32, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Yiannakopoulou, E. Pharmacogenomics and Opioid Analgesics: Clinical Implications. Int. J. Genomics 2015, 2015, e368979. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, P.H.; Stamer, U.M.; Landau, R. Pharmacogenomic considerations in opioid analgesia. Pharm. Pers. Med. 2012, 5, 73–87. [Google Scholar] [Green Version]
- Neighbors, D.M.; Bell, T.J.; Wilson, J.; Dodd, S.L. Economic evaluation of the fentanyl transdermal system for the treatment of chronic moderate to severe pain. J. Pain Symptom Manag. 2001, 21, 129–143. [Google Scholar] [CrossRef]
- Radbruch, L.; Lehmann, K.; Gockel, H.-H.; Neighbors, D.; Nuyts, G. Costs of opioid therapy for chronic nonmalignant pain in Germany: an economic model comparing transdermal fentanyl (Durogesic) with controlled-release morphine. Eur. J. Health Econ. 2002, 3, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Greiner, W.; Lehmann, K.; Earnshaw, S.; Bug, C.; Sabatowski, R. Economic evaluation of Durogesic in moderate to severe, nonmalignant, chronic pain in Germany. Eur. J. Health Econ. 2006, 7, 290–296. [Google Scholar] [CrossRef]
- National Collaborating Centre for Cancer (UK). Opioids in Palliative Care: Safe and Effective Prescribing of Strong Opioids for Pain in Palliative Care of Adults; National Institute for Health and Clinical Excellence: London, UK, 2012.
- Wendt, F.R.; Sajantila, A.; Budowle, B. Predicted activity of UGT2B7, ABCB1, OPRM1, and COMT using full-gene haplotypes and their association with the CYP2D6-inferred metabolizer phenotype. Forensic Sci. Int. Genet. 2018, 33, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaye, A.D.; Garcia, A.J.; Hall, O.M.; Jeha, G.M.; Cramer, K.D.; Granier, A.L.; Kallurkar, A.; Cornett, E.M.; Urman, R.D. Update on the pharmacogenomics of pain management. Pharm. Pers. Med. 2019, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Paix, A.; Coleman, A.; Lees, J.; Grigson, J.; Brooksbank, M.; Thorne, D.; Ashby, M. Subcutaneous fentanyl and sufentanil infusion substitution for morphine intolerance in cancer pain management. Pain 1995, 63, 263–269. [Google Scholar] [CrossRef]
- White, C.; Hardy, J.; Boyd, A.; Hall, A. Subcutaneous sufentanil for palliative care patients in a hospital setting. Palliat. Med. 2008, 22, 89–90. [Google Scholar] [CrossRef]
- Sande, T.A.; Laird, B.J.A.; Fallon, M.T. The use of opioids in cancer patients with renal impairment-a systematic review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2017, 25, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Casuccio, A.; Agnello, A.; Serretta, R.; Calderone, L.; Barresi, L. Morphine versus methadone in the pain treatment of advanced-cancer patients followed up at home. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998, 16, 3656–3661. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Robles, C.R.; Degnan, M.; Candiotti, K.A. Pain and genetics. Curr. Opin. Anaesthesiol. 2012, 25, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Koyyalagunta, D.; Bruera, E.; Solanki, D.R.; Nouri, K.H.; Burton, A.W.; Toro, M.P.; Bruel, B.M.; Manchikanti, L. A systematic review of randomized trials on the effectiveness of opioids for cancer pain. Pain Physician 2012, 15, ES39–ES58. [Google Scholar] [PubMed]
- Kim, E.S. Oxycodone/Naloxone Prolonged Release: A Review in Severe Chronic Pain. Clin. Drug Investig. 2017, 37, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Kim, T.W.; Kang, J.-H.; Kim, J.-S.; Ahn, J.-S.; Kim, S.-Y.; Yun, H.-J.; Eum, Y.-J.; Koh, S.A.; Kim, M.K.; et al. Efficacy and safety of controlled-release oxycodone/naloxone versus controlled-release oxycodone in Korean patients with cancer-related pain: A randomized controlled trial. Chin. J. Cancer 2017, 36, 74. [Google Scholar] [CrossRef]
- Muralidharan, A.; Smith, M.T. Pain, analgesia and genetics. J. Pharm. Pharmacol. 2011, 63, 1387–1400. [Google Scholar] [CrossRef]
- Ting, S.; Schug, S. The pharmacogenomics of pain management: Prospects for personalized medicine. J. Pain Res. 2016, 9, 49–56. [Google Scholar]
- Mercadante, S. The role of tapentadol as a strong opioid in cancer pain management: A systematic and critical review. Curr. Med. Res. Opin. 2017, 33, 1965–1969. [Google Scholar] [CrossRef]
- Palmer, S.N.; Giesecke, N.M.; Body, S.C.; Shernan, S.K.; Fox, A.A.; Collard, C.D. Pharmacogenetics of Anesthetic and Analgesic Agents. J. Am. Soc. Anesthesiol. 2005, 102, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Dobosz, Ł.; Kaczor, M.; Stefaniak, T.J. Pain in pancreatic cancer: Review of medical and surgical remedies. ANZ J. Surg. 2016, 86, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Analgesics Mixed with Pharmacogenomics: The Pain of It all. Available online: http://www.lateralmag.com/columns/gene-dosage/analgesics-mixed-with-pharmacogenomics-the-pain-of-it-all (accessed on 12 November 2016).
- Hussain, M.; Javeed, A.; Ashraf, M.; Al-Zaubai, N.; Stewart, A.; Mukhtar, M.M. Non-steroidal anti-inflammatory drugs, tumour immunity and immunotherapy. Pharmacol. Res. 2012, 66, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Cregg, R.; Russo, G.; Gubbay, A.; Branford, R.; Sato, H. Pharmacogenetics of analgesic drugs. Br. J. Pain 2013, 7, 189–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lussier, D.; Huskey, A.G.; Portenoy, R.K. Adjuvant analgesics in cancer pain management. Oncologist 2004, 9, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; McNicol, E.D.; Bell, R.F.; Carr, D.B.; McIntyre, M.; Wee, B. Oral paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst. Rev. 2017, 7, 1–39. [Google Scholar]
- Fuller, R.W.; Snoddy, H.D. Evaluation of nefopam as a monoamine uptake inhibitor in vivo in mice. Neuropharmacology 1993, 32, 995–999. [Google Scholar] [CrossRef]
- Gray, A.M.; Nevinson, M.J.; Sewell, R.D. The involvement of opioidergic and noradrenergic mechanisms in nefopam antinociception. Eur. J. Pharmacol. 1999, 365, 149–157. [Google Scholar] [CrossRef]
- Hunskaar, S.; Fasmer, O.B.; Broch, O.J.; Hole, K. Involvement of central serotonergic pathways in nefopam-induced antinociception. Eur. J. Pharmacol. 1987, 138, 77–82. [Google Scholar] [CrossRef]
- Kim, S.Y.; Huh, K.H.; Roh, Y.H.; Oh, Y.J.; Park, J.; Choi, Y.S. Nefopam as an adjunct to intravenous patient-controlled analgesia after renal transplantation: A randomised trial. Acta Anaesthesiol. Scand. 2015, 59, 1068–1075. [Google Scholar] [CrossRef]
- Girard, P.; Chauvin, M.; Verleye, M. Nefopam analgesia and its role in multimodal analgesia: A review of preclinical and clinical studies. Clin. Exp. Pharmacol. Physiol. 2016, 43, 3–12. [Google Scholar] [CrossRef]
- Na, H.-S.; Oh, A.-Y.; Koo, B.-W.; Lim, D.-J.; Ryu, J.-H.; Han, J.-W. Preventive Analgesic Efficacy of Nefopam in Acute and Chronic Pain After Breast Cancer Surgery. Medicine (Baltimore) 2016, 95, e3705. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.-Y.; Kwon, J.-Y.; Lee, D.-W.; Kim, E.; Kim, T.-K.; Kim, H.-K. A Randomized Clinical Trial of Nefopam versus Ketorolac Combined with Oxycodone in Patient-Controlled Analgesia after Gynecologic Surgery. Int. J. Med. Sci. 2015, 12, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, J.; Stamer, U.M.; Remi, C.; Voltz, R.; Bausewein, C.; Sabatowski, R.; Wirz, S.; Müller-Mundt, G.; Simon, S.T.; Pralong, A.; et al. Metamizole/dipyrone for the relief of cancer pain: A systematic review and evidence-based recommendations for clinical practice. Palliat. Med. 2017, 31, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Hearn, L.; Derry, S.; Moore, R.A. Single dose dipyrone (metamizole) for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2016, 4, CD011421. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Barutell, C.; Rull, M.; Gálvez, R.; Pallarés, J.; Vidal, F.; Aliaga, L.; Moreno, J.; Puerta, J.; Ortiz, P. Efficacy and tolerance of oral dipyrone versus oral morphine for cancer pain. Eur. J. Cancer 1994, 30, 584–587. [Google Scholar] [CrossRef]
- Brito, B.E.; Vazquez, E.; Taylor, P.; Alvarado, Y.; Vanegas, H.; Millan, A.; Tortorici, V. Antinociceptive effect of systemically administered dipyrone (metamizol), magnesium chloride or both in a murine model of cancer. Eur. J. Pain Lond. Engl. 2017, 21, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.-D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Jones, S. Adjuvant analgesics in cancer pain: A review. Am. J. Hosp. Palliat. Care 2012, 29, 70–79. [Google Scholar] [CrossRef]
- Chwistek, M. Recent advances in understanding and managing cancer pain. F1000Research 2017, 6, 945. [Google Scholar] [CrossRef]
- Caraceni, A.; Zecca, E.; Martini, C.; Pigni, A.; Bracchi, P. Gabapentin for breakthrough pain due to bone metastases. Palliat. Med. 2008, 22, 392–393. [Google Scholar] [CrossRef]
- Hamal, P.K.; Shrestha, A.B.; Shrestha, R.R. Efficacy of Preemptive Gabapentin for Lower Extremity Orthopedic surgery under Subarachnoid Block. JNMA J. Nepal Med. Assoc. 2015, 53, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.S.; Ilfeld, B.M. An Evidence-Based Review of the Efficacy of Perioperative Analgesic Techniques for Breast Cancer-Related Surgery. Pain Med. Malden Mass 2017, 18, 1344–1365. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.M.; O’Hara, E. Pregabalin has opioid-sparing effects following augmentation mammaplasty. Aesthet. Surg. J. 2008, 28, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Song, J.W.; Park, B.; Park, S.; An, Y.J.; Shim, Y.H. Pregabalin reduces post-operative pain after mastectomy: A double-blind, randomized, placebo-controlled study. Acta Anaesthesiol. Scand. 2011, 55, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.K.; Mathew, P.J.; Yaddanapudi, S.; Sehgal, S. A single dose of preoperative gabapentin for pain reduction and requirement of morphine after total mastectomy and axillary dissection: Randomized placebo-controlled double-blind trial. J. Postgrad. Med. 2009, 55, 257–260. [Google Scholar] [PubMed]
- Bugan, I.; Karagoz, Z.; Altun, S.; Djamgoz, M.B.A. Gabapentin, an Analgesic Used Against Cancer-Associated Neuropathic Pain: Effects on Prostate Cancer Progression in an In Vivo Rat Model. Basic Clin. Pharmacol. Toxicol. 2016, 118, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rajagopal, M.R.; Palat, G.; Singh, C.; Haji, A.G.; Jain, D. A phase II pilot study to evaluate use of intravenous lidocaine for opioid-refractory pain in cancer patients. J. Pain Symptom Manag. 2009, 37, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Fassoulaki, A.; Sarantopoulos, C.; Melemeni, A.; Hogan, Q. EMLA reduces acute and chronic pain after breast surgery for cancer. Reg. Anesth. Pain Med. 2000, 25, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Arcuri, E.; Tirelli, W.; Casuccio, A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: A randomized, controlled, double-blind, crossover, double-dose study. J. Pain Symptom Manag. 2000, 20, 246–252. [Google Scholar] [CrossRef]
- Jonkman, K.; van de Donk, T.; Dahan, A. Ketamine for cancer pain: What is the evidence? Curr. Opin. Support. Palliat. Care 2017, 11, 88–92. [Google Scholar] [CrossRef]
- Hardy, J.; Quinn, S.; Fazekas, B.; Plummer, J.; Eckermann, S.; Agar, M.; Spruyt, O.; Rowett, D.; Currow, D.C. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3611–3617. [Google Scholar] [CrossRef]
- Salas, S.; Frasca, M.; Planchet-Barraud, B.; Burucoa, B.; Pascal, M.; Lapiana, J.-M.; Hermet, R.; Castany, C.; Ravallec, F.; Loundou, A.; et al. Ketamine analgesic effect by continuous intravenous infusion in refractory cancer pain: Considerations about the clinical research in palliative care. J. Palliat. Med. 2012, 15, 287–293. [Google Scholar] [CrossRef]
- Ishizuka, P.; Garcia, J.B.S.; Sakata, R.K.; Issy, A.M.; Mülich, S.L. Assessment of oral S+ ketamine associated with morphine for the treatment of oncologic pain. Rev. Bras. Anestesiol. 2007, 57, 19–31. [Google Scholar] [PubMed]
- Birdsall, S.M.; Birdsall, T.C.; Tims, L.A. The Use of Medical Marijuana in Cancer. Curr. Oncol. Rep. 2016, 18, 40. [Google Scholar] [CrossRef]
- Romero-Sandoval, E.A.; Kolano, A.L.; Alvarado-Vázquez, P.A. Cannabis and Cannabinoids for Chronic Pain. Curr. Rheumatol. Rep. 2017, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Guzman, M. Cannabis in cancer care. Clin. Pharmacol. Ther. 2015, 97, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Paice, J.A.; Wallace, M. Pain and Opioids in Cancer Care: Benefits, Risks, and Alternatives. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 705–713. [Google Scholar] [CrossRef]
- Tateo, S. State of the evidence: Cannabinoids and cancer pain-A systematic review. J. Am. Assoc. Nurse Pract. 2017, 29, 94–103. [Google Scholar] [CrossRef]
- Häuser, W.; Fitzcharles, M.-A.; Radbruch, L.; Petzke, F. Cannabinoids in Pain Management and Palliative Medicine. Dtsch. Ärztebl. Int. 2017, 114, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, J.L. Medical marijuana for cancer. CA Cancer J. Clin. 2015, 65, 109–122. [Google Scholar] [CrossRef]
- Hilliard, N.; Brown, S.; Mitchinson, S. A case report of dexmedetomidine used to treat intractable pain and delirium in a tertiary palliative care unit. Palliat. Med. 2015, 29, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Seymore, R.J.; Manis, M.M.; Coyne, P.J. Dexmedetomidine Use in a Case of Severe Cancer Pain. J. Pain Palliat. Care Pharmacother. 2019, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mupamombe, C.T.; Luczkiewicz, D.; Kerr, C. Dexmedetomidine as an Option for Opioid Refractory Pain in the Hospice Setting. J. Palliat. Med. 2019, 22, 1–4. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, J.; Wang, Q.; Xu, M. The antinociceptive effect of systemic administration of a combination of low-dose tramadol and dexmedetomidine in a rat model of bone cancer pain. Eur. J. Anaesthesiol. 2014, 31, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Mohta, M.; Kalra, B.; Sethi, A.K.; Kaur, N. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. J. Anesth. 2016, 30, 252–260. [Google Scholar] [CrossRef]
- Webster, L.R.; Belfer, I. Pharmacogenetics and Personalized Medicine in Pain Management. Clin. Lab. Med. 2016, 36, 493–506. [Google Scholar] [CrossRef]
- Ruano, G.; Kost, J.A. Fundamental Considerations for Genetically-Guided Pain Management with Opioids Based on CYP2D6 and OPRM1 Polymorphisms. Pain Physician 2018, 21, E611–E621. [Google Scholar]
- Owusu Obeng, A.; Hamadeh, I.; Smith, M. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacotherapy 2017, 37, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.R.; Riley, J.; Taegetmeyer, A.B.; Sato, H.; Gretton, S.; du Bois, R.M.; Welsh, K.I. Genetic variation and response to morphine in cancer patients: Catechol-O-methyltransferase and multidrug resistance-1 gene polymorphisms are associated with central side effects. Cancer 2008, 112, 1390–1403. [Google Scholar] [CrossRef]
- Ecimovic, P.; Murray, D.; Doran, P.; McDonald, J.; Lambert, D.G.; Buggy, D.J. Direct effect of morphine on breast cancer cell function in vitro: Role of the NET1 gene. Br. J. Anaesth. 2011, 107, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, J.; Schmidt, H.; Tzvetkov, M.; Keulen, J.-T.H.A.; Lötsch, J.; Roots, I.; Brockmöller, J. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharm. J. 2007, 7, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Udoji, M.A.; Trescot, A. Genetic Testing for Opioid Pain Management: A Primer. Pain Ther. 2017, 6, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.A.; Kuo, Y.-F.; Adhikari, D.; Baillargeon, J.; Goodwin, J.S. Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiol. Drug Saf. 2018, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Oldenmenger, W.H.; Lieverse, P.J.; Janssen, P.J.J.M.; Taal, W.; van der Rijt, C.C.D.; Jager, A. Efficacy of opioid rotation to continuous parenteral hydromorphone in advanced cancer patients failing on other opioids. Support. Care Cancer 2012, 20, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Takashina, Y.; Naito, T.; Mino, Y.; Yagi, T.; Ohnishi, K.; Kawakami, J. Impact of CYP3A5 and ABCB1 gene polymorphisms on fentanyl pharmacokinetics and clinical responses in cancer patients undergoing conversion to a transdermal system. Drug Metab. Pharmacokinet. 2012, 27, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Oosten, A.W.; Matic, M.; van Schaik, R.H.; Look, M.P.; Jongen, J.L.; Mathijssen, R.H.; van der Rijt, C.C. Opioid treatment failure in cancer patients: The role of clinical and genetic factors. Pharmacogenomics 2016, 17, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E.; Palmer, J.L.; Bosnjak, S.; Rico, M.A.; Moyano, J.; Sweeney, C.; Strasser, F.; Willey, J.; Bertolino, M.; Mathias, C.; et al. Methadone versus morphine as a first-line strong opioid for cancer pain: A randomized, double-blind study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kantelip, J.-P.; Gerritsen-van Schieveen, P.; Davani, S. Interindividual variability of methadone response: Impact of genetic polymorphism. Mol. Diagn. Ther. 2008, 12, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Moulin, D.E.; Rogers, A.; Inturrisi, C.E.; Foley, K.M. I.v. infusion of opioids for cancer pain: Clinical review and guidelines for use. Cancer Treat. Rep. 1986, 70, 575–581. [Google Scholar] [PubMed]
- Clarke, T.-K.; Crist, R.C.; Ang, A.; Ambrose-Lanci, L.M.; Lohoff, F.W.; Saxon, A.J.; Ling, W.; Hillhouse, M.P.; Bruce, R.D.; Woody, G.; et al. Genetic variation in OPRD1 and the response to treatment for opioid dependence with buprenorphine in European-American females. Pharm. J. 2014, 14, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sittl, R. Transdermal buprenorphine in cancer pain and palliative care. Palliat. Med. 2006, 20 (Suppl. 1), s25–s30. [Google Scholar]
- Naito, T.; Takashina, Y.; Yamamoto, K.; Tashiro, M.; Ohnishi, K.; Kagawa, Y.; Kawakami, J. CYP3A5*3 affects plasma disposition of noroxycodone and dose escalation in cancer patients receiving oxycodone. J. Clin. Pharmacol. 2011, 51, 1529–1538. [Google Scholar] [CrossRef]
- Arbaiza, D.; Vidal, O. Tramadol in the Treatment of Neuropathic Cancer Pain. Available online: http://www.medscape.com/viewarticle/550883 (accessed on 4 September 2019).
- Galiè, E.; Villani, V.; Terrenato, I.; Pace, A. Tapentadol in neuropathic pain cancer patients: A prospective open label study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017, 38, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Ahmed, E.; Keilson, Y.Y. Cancer pain management: Use of acetaminophen and nonsteroidal antiinflammatory drugs. UpToDate 2019, 18, 1–17. [Google Scholar]
- Eisenberg, E.; Berkey, C.S.; Carr, D.B.; Mosteller, F.; Chalmers, T.C. Efficacy and safety of nonsteroidal antiinflammatory drugs for cancer pain: A meta-analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1994, 12, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Stanley, L.A. Chapter 27—Drug Metabolism. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 527–545. ISBN 978-0-12-802104-0. [Google Scholar]
- Langman, L.J.; Dasgupta, A. Pharmacogenomics in Clinical Therapeutics; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-119-95958-8. [Google Scholar]
- Manworren, R.C.B. Multimodal pain management and the future of a personalized medicine approach to pain. AORN J. 2015, 101, 308–314. [Google Scholar] [CrossRef] [PubMed]


| Analgesics | Study Type | Genetic Variants | Side Effect | References |
|---|---|---|---|---|
| Morphine | Non-randomized clinical trial | Multidrug resistance-1 gene (MDR-1) | Moderate or severe drowsiness and confusion or hallucinations. | [94] |
| Catechol-O-methyltransferase (COMT) enzyme | ||||
| Single nucleotide polymorphisms (SNPs) in intron 1 | ||||
| In vitro study- breast cancer cell lines | NET1 gene expression (mediating the direct effect of morphine on breast cancer cell migration) | [95] | ||
| Codeine | Non-randomized clinical trial | CYP2D6 gene | Sedation, addiction, dizziness and constipation | [96] |
| Hydrocodone | Observational study | CYP2D6 gene | Dizziness and constipation | [97,98] |
| Hydromorphone | Non-randomized clinical trial | CYP2D6 gene | Dizziness and constipation | [97,99] |
| Fentanyl | Non-randomized clinical trial | CYP3A5 and ABCB1 gene polymorphisms | Dry mouth, wheal and flare | [100] |
| Observational study | Genetic variants rs12948783 (RHBDF2) and rs7016778 (OPRK1) | [101] | ||
| Methadone | Randomized double-blind study | ABCB1, OPRM1 gene polymorphisms | Constipation, nausea, dizziness and delirium | [102,103] |
| Levorphanol | Non-randomized clinical trial | - | Nausea and vomiting | [104] |
| Buprenorphine | Non-randomized clinical trial | Polymorphisms in OPRD1 | Dizziness, dry mouth, thirst and nausea | [105,106] |
| Oxycodone | Non-randomized clinical trial | CYP3A5 | Nausea, vomiting, constipation, lightheadedness, dizziness or drowsiness | [107] |
| Tramadol | Randomized double-blind placebo controlled cross over study | CYP2D6 | Dizziness, headache, drowsiness, nausea, vomiting, constipation, lack of energy, sweating and dry mouth | [108] |
| Tapentadol | Non-randomized clinical trial | No genetic variation | Nausea, vomiting, constipation, fatigue, dizziness, sleepiness, drowsiness and dry mouth | [109] |
| Paracetamol (Acetaminophen) | Randomized double-blind placebo controlled parallel group study | COX-3 | Low fever with nausea, stomach pain and loss of appetite | [50] |
| Non-steroidal Anti-Inflammatory Drugs (NSAID) | Randomized, double-blind, placebo-controlled | COX-1/COX-2 | Stomach pain, heartburn, stomach ulcers, a tendency to bleed, headaches, dizziness and ringing in the ears | [110,111] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
V. Subramaniam, A.; Salem Yehya, A.H.; Oon, C.E. Molecular Basis of Cancer Pain Management: An Updated Review. Medicina 2019, 55, 584. https://doi.org/10.3390/medicina55090584
V. Subramaniam A, Salem Yehya AH, Oon CE. Molecular Basis of Cancer Pain Management: An Updated Review. Medicina. 2019; 55(9):584. https://doi.org/10.3390/medicina55090584
Chicago/Turabian StyleV. Subramaniam, Ayappa, Ashwaq Hamid Salem Yehya, and Chern Ein Oon. 2019. "Molecular Basis of Cancer Pain Management: An Updated Review" Medicina 55, no. 9: 584. https://doi.org/10.3390/medicina55090584