Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria
Abstract
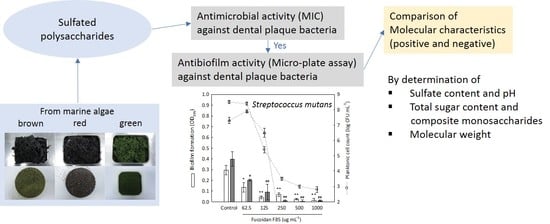
:1. Introduction
2. Results and Discussion
2.1. Isolation Yield of Sulfated Polysaccharides
2.2. Chemical Properties of Sulfated Polysaccharides
2.3. Antimicrobial Screening of Sulfated Polysaccharides
2.4. Antibiofilm Screening of Fucoidan F85 against Selected Dental Plaque Bacteria
2.5. Time-Coursed Inhibitory Effect of Fucoidan F85 on the Growth of S. mutans
2.6. Inhibitory Effect of Fucoidan F85 according to the Treatment Time on the Biofilm Formation of S. mutans
2.7. Composite Monosaccharides and Molecular Weight Estimation
3. Materials and Methods
3.1. Materials
3.2. Isolation of Sulfated Polysaccharide
3.3. Determination of pH, Total Sugar, and Sulfate Contents
3.4. Microorganisms and Culturing Condition
3.5. Minimum Inhibitory Concentration
3.6. Antibiofilm Activity and Planktonic Cell Count
3.7. Time-Coursed Inhibitory Effect of Fucoidan F85 against S. mutans
3.8. Inhibitory Effect of Fucoidan F85 According to the Treatment Time on the Biofilm Formation of S. Mutans
3.9. Composite Monosaccharides
3.10. Molecular Weight
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, T.; Ólafsdóttir, G.; Jónsdóttir, R.; Kristinsson, H.G.; Johannsson, R. Functional and nutraceutical ingredients from marine macroalgae. In Handbook of Seafood Quality, Safety and Health Applications; Alasalvar, C., Miyashita, K., Shahidi, F., Wanasundara, U., Eds.; Blackwell Publishing Ltd.: New York, NY, USA, 2011; pp. 508–521. ISBN 9781405180702. [Google Scholar]
- De Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Arvinda Swamy, M.L. Marine algal sources for treating bacterial diseases. In Marine Medicinal Foods Implications and Applications, Macro and Microalgae; Kim, S.K., Ed.; Elsevier Inc.: Waltham, MA, USA, 2011; Volume 64, pp. 71–84. ISBN 9780123876690. [Google Scholar]
- Jun, J.Y.; Nakajima, S.; Yamazaki, K.; Kawai, Y.; Yasui, H.; Konishi, Y. Isolation of antimicrobial agent from the marine algae Cystoseira hakodatensis. Int. J. Food Sci. Technol. 2015, 50, 871–877. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Walsron, D.S.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. An examination of the potential of seaweed extracts as functional ingredients in milk. Int. J. Dairy Technol. 2014, 67, 182–192. [Google Scholar] [CrossRef]
- Yamashita, S.; Sugita-Konishi, Y.; Shimizu, M. In vitro bacteriostatic effects of dietary polysaccharides. Food Sci. Technol. Res. 2001, 7, 262–264. [Google Scholar] [CrossRef]
- Li, L.Y.; Li, L.Q.; Guo, C.H. Evaluation of in vitro antioxidant and antibacterial activities of Laminaria japonica polysaccharides. J. Med. Plants Res. 2010, 4, 2194–2198. [Google Scholar]
- Pierre, G.; Sopena, V.; Juin, C.; Mastouri, A.; Graber, M.; Maugard, T. Antibacterial activity of a sulfated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2011, 16, 937–945. [Google Scholar] [CrossRef]
- Marudhupandi, T.; Kumar, T.T.A. Antibacterial effect of fucoidan from Sargassum wightii against the chosen human bacterial pathogens. Int. Curr. Pharm. J. 2013, 2, 156–158. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, M.R.; Choi, S.M.; Na, S.S.; Cha, J.D. Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch. Oral Biol. 2013, 58, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6 (Suppl. S1), S14. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, E.; Biggs, C.A. Mechanisms of bacillus cereus biofilm formation: An investigation of the physicochemical characteristics of cell surfaces and extracellular proteins. Appl. Microbiol. Biotechnol. 2011, 89, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Shakila, R.J.; Jawahar, P.; Srinivasan, A. Influence of species, geographic location, seasonal variation and extraction method on the fucoidan yield of the brown seaweeds of Gulf of Mannar, India. Indian J. Pharm. Sci. 2017, 79, 65–71. [Google Scholar] [CrossRef]
- Mabeau, S.; Kloareg, B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcate and Laminaria digitate. J. Exp. Bot. 1987, 38, 1573–1580. [Google Scholar] [CrossRef]
- Do, J.R.; Kim, E.M.; Koo, J.G.; Jo, K.S. Dietary fiber contents of marine algae and extraction condition of the fiber. J. Korean Fish. Soc. 1997, 30, 291–296. [Google Scholar]
- Koo, J.G.; Jo, K.S.; Do, J.R.; Woo, S.J. Isolation and purification of fucoidans from Laminaria religiose and Undaria pinnatifida in Korea. J. Korean Fish. Soc. 1995, 28, 227–236. [Google Scholar]
- Kim, B.M.; Park, J.H.; Kim, D.S.; Kim, Y.M.; Jun, J.Y.; Jeong, I.H.; Chi, Y.M. Effects of the polysaccharide from the sporophyll of brown alga Undaria pinnatifida on serum lipid profile and fat tissue accumulation in rats fed a high-fat diet. J. Food Sci. 2016, 81, H1840–H1845. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.L.; Yang, C.; Kim, S.M.; You, S.G. Molecular characterization and biological activities of water soluble sulfated polysaccharides from Enteromorpha prolifera. Food Sci. Biotechnol. 2010, 19, 525–533. [Google Scholar] [CrossRef]
- Davidson, P.M.; Naidu, A.S. Phyto-phenol. In Natural Food Antimicrobial Systems; Naidu, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 265–294. ISBN 9780849320477. [Google Scholar]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Somani, B.L.; Khanade, J.; Sinha, R. A modified anthrone-sulfuric acid method for the determination of fructose in the presence of certain proteins. Anal. Biochem. 1987, 167, 327–330. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard, 7th ed.; Document M7-A7; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef] [PubMed]



| Origin | Yield | Total Sugar | Mineral | pH † | Sulfate | ||||
|---|---|---|---|---|---|---|---|---|---|
| (%, dry) | (%, dry) | (%, dry) | (%, dry) | ||||||
| Brown algae | |||||||||
| Fucus vesiculosus (F85) * | Nt | ‡ | 83.5 | ±1.9 | 2.2 | ±0.6 | 5.31 | 14.0 | ±2.7 |
| Fucus vesiculosus (F95) * | Nt | 90.5 | ±1.2 | Nt | 9.46 | 7.5 | ±2.1 | ||
| Macrocytis pyrifera (M85) * | Nt | 82.1 | ±2.3 | Nt | 4.93 | 11.4 | ±0.4 | ||
| Undaria pinnatifida (U95) * | Nt | 89.5 | ±1.2 | Nt | 6.05 | 10.5 | ±2.1 | ||
| Hizikia fusiforme | 3.7 | ±0.6 | 65.2 | ±0.7 | 6.8 | ±3.1 | 7.14 | 6.5 | ±1.4 |
| Kjellmaniella crassifolia | 3.0 | ±0.4 | 75.1 | ±2.1 | 6.9 | ±0.2 | 7.81 | 8.6 | ±2.3 |
| Laminaria japonica | 2.8 | ±0.3 | 62.3 | ±3.2 | 7.1 | ±0.4 | 7.73 | 6.4 | ±0.6 |
| Sargassum honeri | 3.5 | ±1.1 | 63.5 | ±0.4 | 6.3 | ±0.3 | 7.43 | 5.2 | ±1.7 |
| Undaria pinnatifida | 2.9 | ±0.3 | 63.0 | ±0.3 | 7.6 | ±0.6 | 7.71 | 7.8 | ±1.4 |
| Green algae | |||||||||
| Capsosiphon fulvescens | 4.5 | ±1.2 | 67.1 | ±1.3 | 5.2 | ±0.3 | 7.65 | 2.5 | ±0.6 |
| Codium fragile | 5.7 | ±0.4 | 58.2 | ±1.1 | 11.4 | ±0.8 | 7.11 | Nd | § |
| Red alga | |||||||||
| Grateloupia flilicina | 28.5 | ±0.9 | 65.1 | ±3.7 | 3.8 | ±0.4 | 6.52 | 3.0 | ±1.2 |
| Bacterial Strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foodborne pathogenic bacteria | ||||||||||||||
| Bacillus cereus KCTC 3624 | NI * | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 25.0 |
| Listeria monocytogenes KCTC 13064 | 250 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 3.1 |
| Staphylococcus aureus subsp. aureus KCTC 3881 | 500 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 25.0 |
| Aeromonas hydrophila subsp. hydrophila KCTC 2358 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | ≥50.0 |
| Escherichia coli KCTC 2441 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 25.0 |
| Salmonella typhimurium KCCM 11862 | NI | NI | NI | NI | NI | 1000 | NI | NI | 1000 | NI | NI | NI | NI | 12.5 |
| Dental plaque bacteria | ||||||||||||||
| Enterococcus faecalis KCTC 5289 | 1000 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 12.5 |
| Streptococcus mutans KCTC 5458 | 125 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 0.8 |
| Streptococcus mutans KCCM 40105 | 250 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 0.8 |
| Streptococcus oralis KCCM 41567 | 500 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 1.6 |
| Streptococcus sobrinus KCTC 5809 | 250 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 1.6 |
| Streptococcus sobrinus KCCM 11898 | 250 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 1.6 |
| Streptococcus sanguinis KCTC 5643 | 500 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 1.6 |
| Lactic acid bacteria | ||||||||||||||
| Lactobacillus acidophilus KCTC 3164 | 500 | NI | NI | NI | NI | NI | NI | NI | 1000 | NI | NI | NI | NI | 3.1 |
| Streptococcus thermophilus KCTC 3658 | 500 | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 1.6 |
| Monosaccharide | F85 (µg mg−1) * | (% Ratio) | F95 (µg mg−1) * | (% Ratio) | ||
|---|---|---|---|---|---|---|
| Fucose | 471.5 | ±5.9 | (87.4) | 521.9 | ±13.9 | (89.7) |
| Rhamnose | † | † | ||||
| Arabinose | † | † | ||||
| Galactose | 33.0 | ±0.5 | (6.1) | 29.1 | ±0.5 | (5.0) |
| Glucose | 10.3 | ±0.6 | (1.9) | Nd | ||
| Mannose | 5.2 | ±0.6 | (1.0) | 11.4 | ±1.1 | (2.0) |
| Xylose | 19.5 | ±0.5 | (3.6) | 19.2 | ±0.3 | (3.3) |
| Fructose | † | † | ||||
| Total | 539.5 | (100.0) | 581.6 | (100.0) | ||
| Heat Treatment | Retention | Mn * | Mw † | Mp ‡ | Mw/Mn § | |
|---|---|---|---|---|---|---|
| Time (min) | (g mol−1) | (g mol−1) | (g mol−1) | |||
| F85 | No | 16.40 | 25,388 | 74,214 | 37,800 | 2.92 |
| Yes | 17.61 | 7834 | 13,875 | 9081 | 1.77 | |
| F95 | No | 16.19 | 17,576 | 62,080 | 48,763 | 3.53 |
| Yes | 16.16 | 38,587 | 76,113 | 50,447 | 1.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, J.-Y.; Jung, M.-J.; Jeong, I.-H.; Yamazaki, K.; Kawai, Y.; Kim, B.-M. Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria. Mar. Drugs 2018, 16, 301. https://doi.org/10.3390/md16090301
Jun J-Y, Jung M-J, Jeong I-H, Yamazaki K, Kawai Y, Kim B-M. Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria. Marine Drugs. 2018; 16(9):301. https://doi.org/10.3390/md16090301
Chicago/Turabian StyleJun, Joon-Young, Min-Jeong Jung, In-Hak Jeong, Koji Yamazaki, Yuji Kawai, and Byoung-Mok Kim. 2018. "Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria" Marine Drugs 16, no. 9: 301. https://doi.org/10.3390/md16090301