The Impact of the Matricaria chamomilla L. Extract, Starch Solution and the Photoinitiator on Physiochemical Properties of Acrylic Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Matricaria chamomilla L. Extract
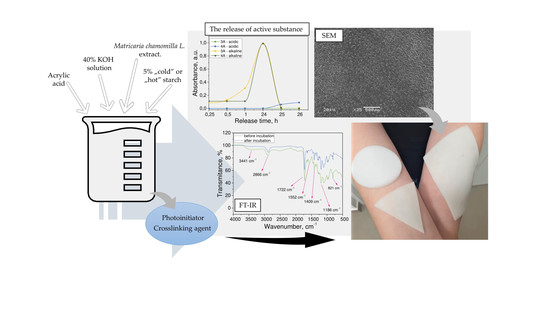
2.3. Synthesis of Hydrogel Polymers
2.4. Methodology of the Research
2.4.1. Investigations on the Sorption Capacity of Hydrogels
2.4.2. Assessment of the Mechanical Properties of Hydrogel Materials
2.4.3. Morphological Analysis of Hydrogels via Scanning Electron Microscopy (SEM)
2.4.4. Studies on the Release of the Active Substance from Developed Hydrogel Matrices
2.4.5. Characterization of the Chemical Structure of Hydrogels via FTIR Technique
2.4.6. Thermal Analysis of Hydrogel
2.4.7. Studies on Cytotoxicity of Hydrogels via MTT Reduction Assay
3. Results and Discussion
3.1. Investigations on the Sorption Capacity of Hydrogels
3.2. Assessment of the Mechanical Properties of Hydrogel Materials
3.3. Morphological Analysis of Hydrogels via Scanning Electron Microscopy (SEM Technique)
3.4. Studies on the Release of the Modifying Agent from the Hydrogel Matrices
3.5. Characterization of the Chemical Structure of Hydrogels via FTIR Technique
3.6. Evaluation of the Thermal Properties of Hydrogels
3.7. Evaluation of Cytotoxicity of Developed Hydrogels via MTT Reduction Assay
4. Conclusions
- The type of the photoinitiator used during the synthesis, the type of the starch solution (temperature of its preparation) and the modifier in the form of Matricaria chamomilla L. extract affected the physicochemical properties of hydrogels.
- The hydrogels modified with Matricaria chamomilla L. extract showed a slightly lower sorption capacity than unmodified polymers. This may result from the presence of numerus organic and inorganic compounds in the extract that may fill free spaces within polymer network.
- The hydrogels prepared by using phenylbis(2,4,6-trimethylbenzoyl) phosphine oxide as a photoinitiator showed lower swelling ability than hydrogels obtained as a result of the photopolymerization initiated by 2-hydroxy-2-methylpropiophenone. This may be caused by the presence of aromatic rings in the structure of phenylbis(2,4,6-trimethylbenzoyl) phosphine oxide, which limits the penetration of absorbed liquid within the polymer network via the so-called steric effect.
- The release of the active substance (i.e., chamomile extract) from the developed hydrogels proceeded only in an alkaline environment.
- Over 90% of chamomile extract was released in the alkaline environment from hydrogels prepared by using phenylbis(2,4,6-trimethylbenzoyl) phosphine oxide as a photoinitiator. Polymers obtained by means of 2-hydroxy-2-methylpropiophenone needed 24 h for the release of such an amount of the active substance.
- Among the hydrogels modified with chamomile extract, the sample prepared by using phenylbis(2,4,6-trimethylbenzoyl) phosphine oxide as a photoinitiator and the starch solution at elevated temperature as a modifier showed the highest stability in the simulated physiological liquid. This was probably caused by the starch gelatinization effect and the complex structure of applied photoinitiator. The rest of analyzed hydrogels degraded in such an environment.
- For the hydrogel obtained by using 2-hydroxy-2-methylpropiophenone as a photoinitiator, the starch solution at the elevated temperature and chamomile extract showed the highest elasticity, as well as the most homogeneous surface morphology.
- The choice of the most beneficial hydrogel composition is strongly influenced by its intended application. For example, the sample showing the highest elasticity may be used as a dressing material. On the other hand, hydrogels which did not degrade as a result of the 30-day incubation in simulated physiological liquid may be used as elements of implants intended for tissue regeneration requiring a longer time to fix. In turn, an easily degradable sample may be applied as a drug carrier which is ultimately biodegradable in the body.
- The hydrogel materials modified with “hot” starch showed higher thermal stability than hydrogels containing “cold” starch. Moreover, the introduction of Matricaria chamomilla L. extract into the hydrogel matrices affects the deterioration of their thermal stability. Nonetheless, in terms of the potential application of developed hydrogels as innovative III generation dressings or drug delivery systems, such a result will not disqualify these materials for the abovementioned applications.
- The hydrogel materials modified with “hot” starch showed no cytotoxicity toward L929 murine fibroblasts. Importantly, hydrogels containing Matricaria chamomilla L. extract increase the cell viability by 15%, thus indicating the cell proliferation. Materials modified with “cold” starch demonstrated cytotoxicity toward tested cell lines; therefore, they cannot be applied for biomedical purposes.
- All of the developed hydrogels showed great application potential and may be successfully widely considered to be applied for biomedical purposes; therefore, in the nearest future, we plan to subject them to more advanced in vitro and in vivo biological analyses.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catani, M.V.; Rinaldi, F.; Tullio, V.; Gasperi, V.; Savini, I. Comparative Analysis of Phenolic Composition of Six Commercially Available Chamomile (Matricaria chamomilla L.) Extracts: Potential Biological Implications. Int. J. Mol. Sci. 2021, 22, 10601. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Potential Application of Peppermint (Mentha piperita L.), German Chamomile (Matricaria chamomilla L.) and Yarrow (Achillea millefolium L.) as Active Fillers in Natural Rubber Biocomposites. Int. J. Mol. Sci. 2021, 22, 7530. [Google Scholar] [CrossRef]
- Abbas, A.M.; Seddik, M.A.; Gahory, A.-A.; Salaheldin, S.; Soliman, W.S. Differences in the Aroma Profile of Chamomile (Matricaria chamomilla L.) after Different Drying Conditions. Sustainability 2021, 13, 5083. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [PubMed]
- Panagiotou, A.; Rossouw, P.E.; Michelogiannakis, D.; Javed, F. Role of Essential Oil-Based Mouthwashes in Controlling Gingivitis in Patients Undergoing Fixed Orthodontic Treatment. A Review of Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 10825. [Google Scholar] [CrossRef]
- Miraj, S.; Alesaeidi, S. A systematic review study of therapeutic effects of Matricaria recuitta chamomile (chamomile). Electron. Physician 2016, 8, 3024–3031. [Google Scholar] [CrossRef] [Green Version]
- Fabian, D.; Juhas, S.; Bukovska, A.; Bujakova, D.; Gresakova, L.; Koppel, J. Anti-Inflammatory Effects of Chamomile Essential Oil in Mice. Slovak J. Anim. Sci. 2011, 44, 111–116. [Google Scholar]
- Bhaskaran, N.; Shukla, S.; Srivastava, J.K.; Gupta, S. Chamomile, an anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. Int. J. Mol. Med. 2010, 26, 935–940. [Google Scholar]
- Srivastava, J.K.; Gupta, S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009, 85, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Sakai, H.; Misawa, M. Effect of sodium azulene sulfonate on capsaicin-induced pharyngitis in rats. Basic Clin. Pharmacol. Toxicol. 2005, 96, 54–59. [Google Scholar] [CrossRef] [PubMed]
- El-Shouny, W.A.E.; El-Zaher, E.H.F.; Khalil, M.A.; El-Salam, O.A. Antimicrobial activity of chamomile acetone extract against some experimentally-induced skin infections in mice. Egypt. J. Environ. Res. EJER 2014, 2, 58–70. [Google Scholar]
- Das, S.; Horváth, B.; Šafranko, S.; Jokić, S.; Széchenyi, A.; Kőszegi, T. Antimicrobial Activity of Chamomile Essential Oil: Effect of Different Formulations. Molecules 2019, 24, 4321. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria recutita. Int. J. Food Prop. 2015, 18, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.S.; Raju, S.S.; Rao, A.V.C. Wound healing activity of Matricaria recutita L. extract. J. Wound Care 2007, 6, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.D.; Marques, M.M.; Bussadori, S.K.; Martins, M.A.T.; Pavesi, V.C.S.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S. Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother. Res. 2009, 23, 274–278. [Google Scholar] [CrossRef]
- Kazemian, H.; Ghafourian, S.; Sadeghifard, N.; Houshmandfar, R.; Badakhsh, B.; Taji, A.; Shavalipour, A.; Mohebi, R.; Ebrahim-Saraie, H.S.; Houri, H.; et al. In vivo Antibacterial and Wound Healing Activities of Roman Chamomile (Chamaemelum nobile). Infect. Disord. Drug Targets 2018, 18, 41–45. [Google Scholar] [CrossRef]
- Wagenbrenner, M.; Mayer-Wagner, S.; Rudert, M.; Holzapfel, B.M.; Weissenberger, M. Combinations of Hydrogels and Mesenchymal Stromal Cells (MSCs) for Cartilage Tissue Engineering—A Review of the Literature. Gels 2021, 7, 217. [Google Scholar] [CrossRef]
- Jayash, S.N.; Cooper, P.R.; Shelton, R.M.; Kuehne, S.A.; Poologasundarampillai, G. Novel Chitosan-Silica Hybrid Hydrogels for Cell Encapsulation and Drug Delivery. Int. J. Mol. Sci. 2021, 22, 12267. [Google Scholar] [CrossRef]
- Koosha, M.; Aalipour, H.; Sarraf Shirazi, M.J.; Jebali, A.; Chi, H.; Hamedi, S.; Wang, N.; Li, T.; Moravvej, H. Physically Crosslinked Chitosan/PVA Hydrogels Containing Honey and Allantoin with Long-Term Biocompatibility for Skin Wound Repair: An In Vitro and In Vivo Study. J. Funct. Biomater. 2021, 12, 61. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Chabria, Y.; Duffy, G.P.; Lowery, A.J.; Dwyer, R.M. Hydrogels: 3D Drug Delivery Systems for Nanoparticles and Extracellular Vesicles. Biomedicines 2021, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.; Yang, X.-B.; Dunne, A.; Florea, L.; Wood, D.; Tronci, G. Thiol-Ene Photo-Click Collagen-PEG Hydrogels: Impact of Water-Soluble Photoinitiators on Cell Viability, Gelation Kinetics and Rheological Properties. Polymers 2017, 9, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.-H.; Lindberg, G.; Soliman, B.; Lim, K.; Woodfield, T.; Narayan, R.J. Effect of Photoinitiator on Precursory Stability and Curing Depth of Thiol-Ene Clickable Gelatin. Polymers 2021, 13, 1877. [Google Scholar] [CrossRef] [PubMed]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef]
- Wiley, K.L.; Ovadia, E.M.; Calo, C.J.; Huber, E.E.; Kloxin, A.M. Rate-based approach for controlling the mechanical properties of ‘thiol-ene’ hydrogels formed with visible light. Polym. Chem. 2019, 10, 4428–4440. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Tyliszczak, B.; Rudnicka, K.; Urbaniak, M.; Michlewska, S.; Królczyk, J.B.; Gajda, P.; Pielichowski, K. Measurement methodology toward determination of structure-property relationships in acrylic hydrogels with starch and nanogold designed for biomedical applications. Measurement 2020, 156, 107608. [Google Scholar] [CrossRef]
- Budianto, E.; Amalia, A. Swelling behavior and mechanical properties of Chitosan-Poly(N-vinyl-pyrrolidone) hydrogels. J. Polym. Eng. 2020, 40, 551–560. [Google Scholar] [CrossRef]
- Kopac, T.; Abrami, M.; Grassi, M.; Rucigaj, A.; Krajnc, M. Polysaccharide-based hydrogels crosslink density equation: A rheological and LF-NMR study of polymer-polymer interactions. Carbohydr. Polym. 2022, 277, 118895. [Google Scholar] [CrossRef]
- Niu, Y.; Zheng, Y.; Fu, X.; Zeng, D.; Liu, H. A novel characterization of starch gelatinization using microscopy observation with deep learning methodology. J. Food Eng. 2022, 327, 111057. [Google Scholar] [CrossRef]
- Haixja, Z.; Zhiguang, C.; Junrong, H.; Huayin, P. Exploration of the process and mechanism of magnesium chloride induced starch gelatinization. Int. J. Biol. Macromol. 2022, 205, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Tao, L.; Jiang, S.; Yan, J.; Hu, S.; Yang, G.; Ma, C.; Cheng, S.; Wang, X.; Yin, J. Highly flexible hydrogel dressing with efficient antibacterial, antioxidative, and wound healing performances. Biomater. Sci. 2022, 10, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, W.D.; Rao, Y.F.; Lu, X.Y.; Gao, J.Q. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Schultz, K.M. Correlation of Bulk Degradation and Molecular Release from Enzymatically Degradable Polymeric Hydrogels. Biomacromolecules 2021, 22, 4489–4500. [Google Scholar] [CrossRef]
- Koev, T.T.; Muñoz-García, J.C.; Iuga, D.; Khimyak, Y.Z.; Warren, F.J. Structural heterogeneities in starch hydrogels. Carbohydr. Polym. 2020, 249, 116834. [Google Scholar] [CrossRef]
- Kalendova, P.; Svoboda, L.; Hroch, J.; Honcova, P.; Drobna, H.; Slang, S. Hydrogels Based on Starch from Various Natural Sources: Synthesis and Characterization. Starch Staerke 2021, 73, 2100051. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch Staerke 2013, 65, 48–60. [Google Scholar] [CrossRef]
- PN-EN ISO Standard 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: In Vitro Cytotoxicity Studies. Comite Europeen de Normalisation: Brussels, Belgium, 2009.














| Sample | Acrylic Acid, mL | 40% KOH Solution, mL | 5% “Cold” Starch v/v, mL | 5% “hot” starch v/v, mL | Matricaria chamomilla L. Extract, mL | Crosslinker *, mL | Photoinitiator A **, mL | Photoinitiator B ***, mL |
|---|---|---|---|---|---|---|---|---|
| 1A | 15 | 17 | 5 | - | - | 5 | 0.25 | - |
| 2A | - | 5 | - | |||||
| 3A | 5 | - | 5 | |||||
| 4A | - | 5 | 5 | |||||
| 1B | 5 | - | - | - | 0.25 | |||
| 2B | - | 5 | - | |||||
| 3B | 5 | - | 5 | |||||
| 4B | - | 5 | 5 |
| Sample | Name of Sample |
|---|---|
| 1A | 5_cold_S/0_MC/Phot_A |
| 2A | 5_hot_S/0_MC/Phot_A |
| 3A | 5_cold_S/5_MC/Phot_A |
| 4A | 5_hot_S/5_MC/Phot_A |
| 1B | 5_cold_S/0_MC/Phot_B |
| 2B | 5_hot_S/0_MC/Phot_B |
| 3B | 5_cold_S/5_MC/Phot_B |
| 4B | 5_hot_S/5_MC/Phot_B |
| Wavenumber, cm−1 | Functional Group |
|---|---|
| 3441 | O-H |
| 2866 | C-H aliphatic |
| 1722 | C=O acidic |
| 1552 | C=C aromatic |
| 1409 | O-H |
| 1186 | C-O |
| 821 | C-H |
| No. | Compound | Structural Formula |
|---|---|---|
| 1. | Starch |  |
| 2. | Acrylic acid |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamroży, M.; Głąb, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Gajda, P.; Tyliszczak, B. The Impact of the Matricaria chamomilla L. Extract, Starch Solution and the Photoinitiator on Physiochemical Properties of Acrylic Hydrogels. Materials 2022, 15, 2837. https://doi.org/10.3390/ma15082837
Jamroży M, Głąb M, Kudłacik-Kramarczyk S, Drabczyk A, Gajda P, Tyliszczak B. The Impact of the Matricaria chamomilla L. Extract, Starch Solution and the Photoinitiator on Physiochemical Properties of Acrylic Hydrogels. Materials. 2022; 15(8):2837. https://doi.org/10.3390/ma15082837
Chicago/Turabian StyleJamroży, Mateusz, Magdalena Głąb, Sonia Kudłacik-Kramarczyk, Anna Drabczyk, Paweł Gajda, and Bożena Tyliszczak. 2022. "The Impact of the Matricaria chamomilla L. Extract, Starch Solution and the Photoinitiator on Physiochemical Properties of Acrylic Hydrogels" Materials 15, no. 8: 2837. https://doi.org/10.3390/ma15082837