Recent Advances in Nanoscale Based Electrocatalysts for Metal-Air Battery, Fuel Cell and Water-Splitting Applications: An Overview
Abstract
:1. Introduction
2. Nanoscale-Based Electrode Catalysts for Metal-Air Battery
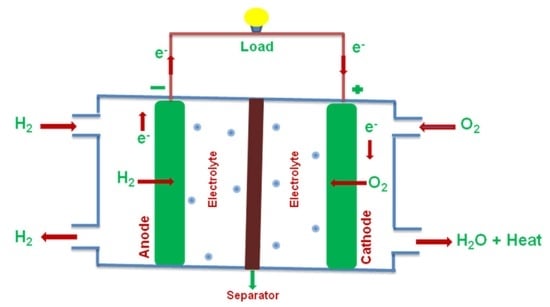
3. Nanoscale Based Electrode Catalysts for Fuel Cells
4. Nanoscale Based Catalysts for Water Splitting Reaction
5. Electrode Stability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage; current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Zhao, D.; Zhuang, Z.; Cao, X.; Zhang, C.; Peng, Q.; Chen, C.; Li, Y. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem. Soc. Rev. 2020, 49, 2215–2264. [Google Scholar] [CrossRef]
- Tabassum, H.; Mahmood, A.; Zhu, B.; Liang, Z.; Zhong, R.; Guo, S.; Zou, R. Recent advances in confining metal-based nanoparticles in to carbon nanotubes for electrochemical energy conversion and storage devices. Energy Environ. Sci. 2019, 12, 2924–2956. [Google Scholar] [CrossRef]
- Goncalves, J.M.; Silva, M.N.T.; Naik, K.K.; Martins, P.R.; Rocha, D.P.; Nossol, E.; Munoz, R.A.A.; Angnes, L.; Rout, C.S. Multifunctional spinel MnCo2O4 based materials for energy storage and conversion; a review on emerging trends, recent developments and future perspectives. J. Mater. Chem. A 2021, 9, 3095–3124. [Google Scholar] [CrossRef]
- Li, X.; Popov, B.N.; Kawahara, T.; Yanagi, H. Recent advances in non-precious metal catalyst for oxygen reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.G.; Qiao, S.Z. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; Cu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.J.; Wang, J.Q.; Hu, J.S.; Wei, Z.; Wan, L.J. Understanding the high active of Fe-N-C electrocatalysts in oxygen reduction; Fe/Fe3C nanoparticles boost the activity of Fe-Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W. Carbon nanomaterials; synthesis, properties and applications in electrochemical sensors and energy conversion systems. Mater. Sci. Eng. B 2021, 272, 115341. [Google Scholar] [CrossRef]
- Fiorani, A.; Merino, J.P.; Zanut, A.; Criado, A.; Valenti, G.; Prato, M.; Paolucci, F. Advanced carbon nanomaterials for electrochemiluminescence biosensor applications. Curr. Opin. Electrochem. 2019, 16, 66–74. [Google Scholar] [CrossRef]
- Majumdar, D. Recent progress in copper sulfide based nanomaterials for high energy supercapacitor applications. J. Electroanal. Chem. 2021, 880, 114825. [Google Scholar] [CrossRef]
- Qin, X.; Huang, Y.; Shen, Y.; Zhao, M.; Gao, X. Porous 3D flower-like bismuthsilicate@nitrogen-doped graphene nanomaterials as a high-efficient catalyst for fuel cell cathode. Ceram. Int. 2019, 45, 24515–24527. [Google Scholar] [CrossRef]
- Benzait, Z.; Yuca, N. Synergistic effect of carbon nanomaterials on a cost-effective coral-like Si/rGO composite for lithium-ion battery applications. Electrochim. Acta 2020, 339, 135917. [Google Scholar] [CrossRef]
- Kuang, M.; Wang, Q.; Ge, H.; Han, P.; Gu, Z.; Al-Enizi, A.M.; Zheng, G. CuCoOx/FeOOH core-shell nanowire as an efficient bifunctional oxygen evolution and reduction catalyst. ACS Energy Lett. 2017, 2, 2498–2505. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, X.; Ding, J.; Hu, W.; Zhong, C.; Lu, J. The challenges in zinc electrodes for rechargeable alkaline zinc-air batteries; obstacles to commercialization. ACS Energy Lett. 2019, 4, 2259–2270. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolytic membrane fuel cells, Technology. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Gao, J.; Wang, E.; Jiang, L.; Anc, L.; Wang, X. An effective hybrid organic/inorganic inhibition for alkaline aluminium-air fuel cells. Electrochim. Acta 2017, 248, 478–485. [Google Scholar] [CrossRef]
- Hoogers, G. Fuel Cell Technology Handbook; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2003. [Google Scholar]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell; recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Merie, G.; Wessling, J.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar]
- Wang, H.F.; Xu, Q. Materials design for rechargeable metal-air batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Shao, L.; Liang, Z.X.; Chen, H.; Song, X.Z.X.; Deng, X.H.; Huo, G.; Kang, X.M.; Wang, L.; Fu, X.Z.; Luo, J.L. CuCo2S4 hollow nanoneedle arrays supported on Ni foams as efficient trifunctional electrocatalysts for overall water splitting and Al-air batteries. J. Alloy. Compd. 2020, 845, 155392. [Google Scholar] [CrossRef]
- Zhang, P.; Zhan, T.; Rong, H.; Feng, Y.; Wen, Y.; Zhao, J.; Wang, L.; Liu, X.; Hou, W. NiFe-coordinated Zeolite imidazolate framework derived trifunctional electrocatalysts for overall water-splitting and zinc-air batteries. J. Colloids Interfaces 2020, 579, 1–11. [Google Scholar] [CrossRef]
- Wang, H.; Lee, H.W.; Deng, Y.; Lu, Z.; Hsu, P.C.; Liu, Y.; Lin, D.; Cui, Y. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water-splitting. Nat. Commun. 2015, 6, 7261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logeshwaran, N.; Ramakrishnan, S.; Chandrasekaan, S.S.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Velusamy, D.B.; Varadhan, P.; He, J.H.; Yoo, D.J. An efficient and durable trifunctional electrocatalyst for zinc-air batteries driven overall water splitting. Appl. Catal. B Environ. 2021, 297, 120405. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Pei, Z.; Huang, Y.; Liang, G.; Mo, F.; Yang, Q.; Su, J.; Gao, Y.; Zapien, J.A.; et al. Single-site active iron-based bi-functional oxygen catalyst for a compressible and rechargeable zinc-air battery. ACS Nano 2018, 12, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF nanocrystals to Co-Nx/C nanorod array electrocatalysts for ORR, OER and Zn-air batteries. Adv. Funct. Mater. 2017, 28, 1704638. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Lei, Z.; Tan, Y.; Wu, W.; Mu, S.; Cheng, N. Defect enriched hollow porous Co-N-doped carbon for oxygen reduction reaction and Zn-air batteries. Carbon 2020, 167, 188–195. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Du, H.; Zhu, J.; Hu, L.; Guo, D. Eu2O3-Cu/NC nanocomposite catalyst with improved oxygen reduction reaction activity for Zn-air batteries. Int. J. Hydrog. Energy 2021, 46, 3974–3983. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Feng, L. A review on advanced FeNi-based catalyst for water splitting reaction. Energy Fuels 2020, 34, 13491–13522. [Google Scholar] [CrossRef]
- Yu, J.; He, Q.; Yang, G.; Zhou, W.; Shao, Z.; Ni, M. Recent advances and prospective in ruthenium-based materials for electrochemical water splitting. ACS Catal. 2019, 9, 9973–10011. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, L.; Shi, J. Anion-containing noble-metal-free bifunctional electrocatalysts for overall water splitting. ACS Catal. 2018, 8, 3688–3707. [Google Scholar] [CrossRef]
- Jiang, Y.; Deing, Y.P.; Liang, R.; Fu, J.; Luo, D.; Li, J.; Zhang, Z.; Hu, Y.; Chen, Z. Multidimensional ordered bi-functional air electrode enable flash reactants shuttling for high-energy flexible Zn-air batteries. Adv. Energy Mater. 2019, 9, 1900911. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-Air Batteries—A Review. Energies 2021, 14, 7373. [Google Scholar]
- Chen, X.; Liu, B.; Zong, C.; Liu, Z.; Liu, J.; Ma, L.; Deng, Y.; Han, X.; Wu, T.; Hu, W.; et al. Ultrathin Co3O4 layers with large contact area on carbon-fibers as a high-performance electrode for flexible zinc-air battery integrated with flexible display. Adv. Energy Mater. 2017, 7, 1700779. [Google Scholar] [CrossRef]
- Liagn, J.; Zhou, R.F.; Chen, X.M.; Jang, Y.H.; Qiao, S.N. Fe-N decorated hybrids of CNTs grown on hierarchically porous carbon for high-performance oxygen reduction. Adv. Mater. 2014, 26, 6074–6079. [Google Scholar]
- Wang, F.; Li, G.; Meng, X.; Xu, S.; Ma, W. One-dimensional Mn3O4/NiCo2S4 nanocomposites as a high-performance bifunctional electrocatalyst for rechargeable liquid/flexible Zn-air, batteries. J. Power Sources 2020, 462, 228162. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Y.; Zhang, T.; Liu, Y.; Qiao, J. Efficient quantum dots anchored nanocomposite for highly active ORR/OER electrocatalyst of advanced metal-air batteries. Nano Energy 2019, 57, 176–185. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Sun, Q.; Pan, Q.; Zhao, N.; Li, Z.; Yang, Y.; Sun, X. Controllable synthesis of Co@CoOx/helical nitrogen-doped carbon nanotubes towards oxygen reduction reaction as binder-free cathodes for Al-air battery. ACS Appl. Mater. Interfaces 2020, 12, 16512–16520. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M.A.; Mele, G. Polyaniline/Zn-phthalocyanines nanocomposite for protecting zinc electrode in Zn-air battery. J. Power Sources 2019, 443, 227264. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Fang, G.; Xie, G.; Liu, X.; Lin, X.; Qiu, H.J. Multi-component spinel metal oxide nanocomposites as high performance bi-functional catalysts in Zn-air batteries. ACS Appl. Energy Mater. 2020, 3, 7710–7718. [Google Scholar]
- Leisegang, T.; Meutzner, F.; Zschornak, M.; Münchgesang, W.; Schmid, R.; Tina, N.; Eremin, R.A.; Kabanov, A.A.; Blatov, V.A.; Meyer, D.C. The Aluminum-Ion Battery: A Sustainable and Seminal Concept? Front. Chem. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc-air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Zahoor, A.; Faizan, R.; Elsaid, K.; Hashmi, S.; Butt, F.A. Synthesis and experimental investigation of α-MnO2/N-RGO nanocomposite for Li-O2 battery applications. Chem. Eng. J. Adv. 2021, 7, 100115. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Li, Z.; Wang, J.; Gregory, D.H.; Chen, J. MWCNTs@MnO2 nanocompsosite cathode integrated with soluble O2-carrier Co-salen in electrolyte for high performance Li-air batteries. Nano Lett. 2017, 17, 2073–2078. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Tang, Z.; Wu, W.; Xi, P.; Liu, D.; Ding, Z.; Chen, X.; Wu, X.; Chen, S. Nanocomposites of Copt-x/Diatomite-C as oxygen reversible electrocatalysts for zinc-air batteries; Diatomite boosted the catalytic for zinc-air batteries; Diatomite boosted the catalytic activity and durability. Electrochim. Acta 2018, 284, 119–127. [Google Scholar] [CrossRef]
- Hao, R.; Ren, J.T.; Lv, X.W.; Li, W.; Liu, Y.P.; Yuan, Z.Y. N-doped porous carbon hollow microspheres encapsulated with iron-based nanocomposites as advanced bi-fucntional catalyst for rechargeable zinc-air battery. J. Energy Chem. 2020, 49, 14–21. [Google Scholar] [CrossRef]
- Lin, C.; Li, X.; Shinde, S.S.; Kim, D.H.; Zhang, H.; Lee, J.H. A long-life rechargeable Zn-air battery based on binary metal carbide armored by nitrogen-doped carbon. ACS Appl. Energy Mater. 2019, 2, 1747–1755. [Google Scholar] [CrossRef]
- Akitha, M.; Elumalai, P. Porous Carbon Networks Decorated with Cobalt on CoFe2O4 as an Air-BreathingElectrode for High-Capacity RechargeableLithium-Air Batteries: Role of Metallic Cobalt Nanoparticles. Chem. Electrochem. 2020, 7, 4188–4200. [Google Scholar]
- Jung, J.W.; Jang, J.S.; Yun, T.G.; Yoon, K.R.; Kim, I.D. 3D nanofibrous air electrode assembled with carbon nanotube bridged hollow Fe2O3 nanoparticles for high performance lithium oxygen batteries. ACS Appl. Mater. Interfaces 2018, 10, 6531–6540. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Zhang, Q.; Liang, Z.; Gu, L.; Guo, W.; Zhu, S.; Zou, R. Metal-organic-framework-derived Fe/Cu-substituted Co nanoparticles embedded in CNTs-grafted carbon polyhedron for Zn-air batteries. Carbon Energy 2020, 2, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Das, C.; Bothra, N.; Sikdar, N.; Das, S.; Pati, S.K.; Maji, T.K. MOF derived Co3O4@Co/NCNT nanocomposite for electrochemical hydrogen evolution, flexible Zinc-air batteries and overall water splitting. Inorg. Chem. 2020, 59, 3160–3170. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Fang, J.; Villa, C.J.; Xu, Y.; Hao, S.; Li, J.; Liu, Y.; Wolverton, C.; Chen, X.; et al. Superior oxygen reduction reaction on phosphorous-doped carbon dots/graphene aerogel for all-solid-state flexible Al-air batteries. Adv. Energy Mater. 2019, 10, 1902736. [Google Scholar] [CrossRef]
- Ali, S.; Khan, T.; Khan, M.; Khan, R.; Hussain, S. Morphological structure and energy storage based study of MoS2ZnO nanocomposite. Mater. Res. Express. 2019, 6, 125087. [Google Scholar] [CrossRef]
- Aiyappa, H.B.; Bhange, S.N.; Sivasankaran, V.P.; Kurungot, S. Single cell fabrication towards realistic evaluation of CNT strong ZIF-derived electrocatalyst as cathode material in alkaline fuel cells and metal-air battery. Chem. Electro. Chem. 2017, 4, 2928–2933. [Google Scholar]
- Prabhakaran, S.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Hierarchical 3D oxygenated cobalt molybdenum selenide nanosheets as robust trifunctional catalyst for water splitting and Zinc-air batteries. Small 2020, 16, 2000797. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.X.; Wang, J.; Zhang, Q.; Meng, F.; Bao, D.; Liu, T.; Yang, X.Y.; Chang, Z.W.; Yan, J.M.; Zhang, X.B. In situ coupling FeM (M = Ni, Co) with nitrogen-doped porous carbon towards highly efficient trifunctional electrocatalyst for overall water splitting and rechargeable Zn-air battery. Adv. Sustain. Sys. 2017, 1, 1700020. [Google Scholar] [CrossRef]
- Chen, B.; He, X.; Yin, F.; Wang, H.; Liu, D.J.; Shi, R.; Chen, J.; Yin, H. MO-Co@N-doped carbon (M = Zn or Co); vital roles of inactive Zn and highly efficient activity towards oxygen reduction/evaluation reaction for rechargeable Zn-air battery. Adv. Funct. Mater. 2017, 27, 1700795. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, H.; Ji, S.; Wang, X.; Liu, Q.; Brett, D.J.L.; Linkov, V.; Wang, R. Mn nanoparticles encapsulated within mesoporous helical N-doped carbon nanotubes as highly active air cathode for Zinc-air batteries. Adv. Sustain. Syst. 2019, 3, 1900085. [Google Scholar] [CrossRef]
- Nam, G.; Park, J.; Choi, M.; Oh, P.; Park, S.; Kim, M.G.; Park, N.; Cho, J.; Lee, J.S. Carbon-coated core-shell Fe-Cu nanoparticles as highly active and durable electrocatalyst for a Zn-air battery. ACS Nano 2015, 9, 6493–6501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lai, Q.; Wang, Y.; Zhu, J.; Liang, Y. Interconnected hierarchically porous Fe, N-co-doped carbon nanofibers as efficient oxygen reduction catalyst for Zn-air batteries. ACS Appl. Mater. Intefaces 2017, 9, 16178–16186. [Google Scholar] [CrossRef]
- Wang, L.; Yue, S.; Zhang, Q.; Zhang, Y.; Li, Y.R.; Lewis, C.S.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Wong, S.S. Morphological and chemical turning of high-energy density metal oxides for lithium-ion battery electrode applications. ACS Energy Lett. 2017, 2, 1465–1478. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Meng, T.; Guo, D.; Wang, S.; Mao, B.; Cao, M. Co9S8@MoS2 core-shell heterostructures as trifunctional electrocatalyst for overall water splitting and Zn-air batteries. ACS Appl. Mater. Interfaces 2017, 10, 1678–1689. [Google Scholar] [CrossRef]
- Moni, P.; Pollachini, M.G.; Wilhelm, M.; Lorenz, J.; Harms, C.; Murshed, M.M.; Rezwan, K. Metal-containing ceramic composite with in situ grown carbon nanotube as a cathode catalyst for anion exchange membrane fuel cells and rechargeable Zinc-air battery. ACS Appl. Energy Mater. 2019, 2, 6078–6086. [Google Scholar] [CrossRef]
- Tan, Z.F.; Zhang, C.; Liu, P.K.; Reed, B.; Zhao, Y.J. Focus on fuel cell system in China. Rev. Sustain. Energy Rev. 2015, 47, 912–923. [Google Scholar]
- Chen, T.W.; Ramachandran, R.; Chen, S.M.; Anushya, G.; Divya Rani, S.; Vinitha, M.; Elumalai, P.; Vasimalai, N. High-Performance-Based Perovskite-Supported Nanocomposite for the Development of Green Energy Device Applications: An Overview. Nanomaterials 2021, 11, 1006. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.I.; Heikkila, M.; Lund, P.D. Advanced low-temperature ceramic nanocomposite fuel cells using ultra high ionic conductivity electrolytes synthesized through freeze-dried method and solid-route. Mater. Today Energy 2017, 5, 338–346. [Google Scholar] [CrossRef]
- Khan, I.; Asghar, M.I.; Lund, P.D.; Basu, S. High conductive (LiNaK)2CO3Ce0.85Sm0.15O2 electrolyte compositions for IT-SOFC applications. Int. J. Hydrog. Energy 2017, 42, 20904–20909. [Google Scholar] [CrossRef]
- Asghar, M.I.; Lund, P.D. Ceramic-carbonate nanocomposite fuel cells. Nanoworld 2016, 3, 7e11. [Google Scholar]
- Asghar, M.I.; Lund, P.D. High performance ceramic nanocomposite fuel cells utilizing LiNiCuZn-oxide anode based on slurry method. Int. J. Hydrog. Energy 2018, 43, 12797–12802. [Google Scholar] [CrossRef]
- Makharia, R.; Kocha, S.S.; Yu, P.T.; Sweikart, M.A.; Gu, W.; Wagner, F.T.; Gasteiger, H.A. Durable PEM Fuel cell Electrode materials: Requirements and Benchmarking Methodologies. ECS Trans. 2006, 1, 3. [Google Scholar] [CrossRef]
- Gouda, M.H.; Gouveia, W.; Afonso, M.L.; Sljukic, B.; El Essawy, N.A.; Nassr, A.B.A.A.; Santos, D.M.F. Poly(vinyl alcohol)-based cross linked ternary polymer blend doped with sulfonated graphene oxide as a sustainable composite membrane for direct borohydride fuel cells. J. Power Sources 2019, 432, 92–101. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elnouby, M.; Aziz, A.N.; Elsayed Youssef, M.; Santos, D.M.F.; Elessawy, N.A. Green and Low-cost Membrane Electrode Assembly for Proton Exchange Membrane Fuel cells: Effect of Double-Layer Electrodes and Gas Diffusion Layer. Front. Mater. Sci. 2020, 6, 337. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.L.; Wang, L.; Chi, B.; Xu, Y.; Zaman, S.; Qi, K.; Liu, H.; Liao, S.; Xia, B.Y. Formation of a Tubular Assembly by Ultrathin Ti0.8Co0.2N Nanosheets as Efficient Oxygen Reduction Electrocatalysts for Hydrogen-/Metal-Air Fuel Cells. ACS Catal. 2018, 8, 8970–8975. [Google Scholar] [CrossRef]
- Ohayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Chen, T.W.; Ramachandran, R.; Chen, S.M.; Anushya, G.; Ramachandran, K. Graphene and Perovskite-Based Nanocomposite for Both Electrochemical and Gas Sensor Applications: An Overview. Sensors 2020, 20, 6755. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, Q.; Gao, J.; Sun, L.; Huo, L.; Zhao, H. Highly electrocatalytic active and durable Fe based perovskite oxygen reduction electrode for solid oxide fuel cells. J. Alloy. Compd. 2021, 858, 158265. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Javanbakht, M.; Askari, M.B.; Hooshyari, K.; Moradi, M.; Beydaghi, H.; Rastgoo-Deylami, M.; Enhessari, M. Novel proton conducting core-shell PAMPS-PVBS@Fe2TiO5 nanoparticles as a reinforcement for SPEEK based membranes. Sci. Rep. 2021, 11, 4926. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Banik, S.; Majumdar, D.; Bhattacharya, S.K. Anodic Oxidation of Butan-1-ol on Reduced Graphene oxide Supported Pd-Ag Nano alloy for Fuel Cell Application. ACS Omega 2019, 4, 4658–4670. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Jiang, S.P. Characterization of High-Temperature Proton-Exchange Membranes Based on Phosphotungstic Acid Functionalized Mesoporous Silica Nanocomposites for Fuel Cells. J. Phys. Chem. C 2011, 115, 11854–11863. [Google Scholar] [CrossRef]
- Uma, T.; Nogami, M. Proton Conducting Gas Electrolyte. Anal. Chem. 2008, 80, 506508. [Google Scholar] [CrossRef]
- Nakanishi, T.; Norisuye, T.; Sato, H.; Takemori, T.; TranCong-Miyata, Q.; Sugimoto, T.; Nomura, S. Studies on Microscopic Structure of Sol-Gel Derived Nanohybrids Containing Hetero polyacid. Macromolecules 2007, 40, 4165–4172. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y.; Shi, J.; Nogami, M. Synthesis and proton conductivity of large-sized crack-free mesostructured phosphorus -oxide doped silica monoliths. Microporous Mesoporous Mater. 2008, 111, 343–349. [Google Scholar] [CrossRef]
- Uma, T.; Nogami, M. Properties of PWA/ZrO2-doped phosphosilicate glass composite membranes for low-temperature H2/O2 fuel cell applications. J. Membr. Sci. 2008, 323, 11. [Google Scholar] [CrossRef]
- Inoue, T.; Uma, T.; Nogami, M.J. Performance of H2/O2 fuel cell using membrane electrolyte of phosphotungstic acid-modified 3-glycidoxypropyl-trimethoxysilanes. J. Member. Sci. 2008, 323, 148–152. [Google Scholar] [CrossRef]
- Uma, T.; Nogami, M. A Novel Glass Membrane for Low Temperature H2/O2 Fuel Cell Electrolytes. Fuel Cells 2007, 7, 279–284. [Google Scholar] [CrossRef]
- Tayyab, Z.; Rehman, S.U.; Shakir, I.; Ajmal Khan, M.; Mushtaq, N.; Alvi, F.; Rauf, S.; Khan, A.; Fatima, M.; Raza, R. Catalytic study of efficient nanocomposites {Ni0.5Zn0.5-xCex-oxides electrodes} for natural gas-fed fuel cells. Mater. Res. Express. 2020, 7, 015508. [Google Scholar] [CrossRef]
- Chen, B.Y.; Liu, S.Q.; Hung, J.Y.; Shiau, T.J.; Wang, Y.M. Reduction of carbon dioxide emission by using microbial fuel cells during wastewater treatment. Aerosol Air Qual. Res. 2013, 13, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Tharali, A.D.; Sain, N.; Osborne, W.J. Microbial fuel cells in bioelectricity production. Front. Life Sci. 2016, 9, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Asghar, M.I.; Lepikko, S.; Patakangas, J.; Halme, J.; Lund, P.D. Comparative analysis of ceramic-carbonate nanocomposite fuel cells using composite GDC/NLC electrolyte with different perovskite structured cathode materials. Front. Chem. Sci. Eng. 2017, 12, 162–173. [Google Scholar] [CrossRef]
- Eksioglu, A.; Arslan, L.C.; Sezen, M.; Ow-Yang, C.W.; Buyukaksoy, A. Formation of Nanocomposite Solid Oxide Fuel Cell Cathodes by Preferential Clustering of Cations from a Single Polymeric Precursor. ACS Appl. Mater. Interfaces 2019, 11, 47904–47916. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, D.B.; Qin, H.; Gao, F.; Inui, H.; Zhu, B. Nanocomposite electrode materials for low temperature solid oxide fuel cells using the ceria-carbonate composite electrolytes. Int. J. Hydrog. Energy 2012, 37, 19351–19356. [Google Scholar] [CrossRef]
- Xiang, Y.; Xin, L.; Hu, J.; Li, C.; Qi, J.; Hou, Y.; Wei, X. Advances in the Applications of Graphene-Based Nanocomposites in Clean Energy Materials. Crystals 2021, 11, 47. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Du, Z.; Swierczek, K.; Li, Y. High performance SmBaMn2O5+δ electrode for symmetrical solid oxide fuel cell. Chem. Mater. 2019, 31, 3784–3793. [Google Scholar] [CrossRef]
- Arif Khan, M.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.Y.; Vedhanarayanan, B.; Lin, S.Y.; Shao, L.D.; Sofer, Z.; Lin, J.Y.; Lin, T.W. Electrodeposited NiSe on a forest of carbon nanotubes as a free-standing electrode for hybrid capacitors and overall water splitting. J. Colloids Interface Sci. 2020, 574, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Huang, J.; Yu, J.; Chen, G.; Hu, W.; Yin, M.; Zhang, R.; Chu, S.; Li, C. Ni3S2 nanosheet flowers decorated with CdS quantum dots as a highly active electrocatalysis electrode for synergistic water splitting. ACS Appl. Mater. Interfaces 2017, 9, 29660–29668. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.R.; Cao, X.; Gao, Q.; Xu, Y.F.; Zheng, Y.R.; Jiang, J.; Yu, S.H. Nitrogen-doped graphene supported CoSe2 nanobelt composite catalyst for efficient water oxidation. ACS Nano 2014, 8, 3970–3978. [Google Scholar] [CrossRef]
- Qin, Q.; Li, P.; Chen, L.; Liu, X. Coupling bimetallic oxides alloys and N-doped carbon nanotubes as tri-functional catalyst for overall water splitting and Zinc-air batteries. ACS Appl. Mater. Interfaces 2018, 10, 39828–39838. [Google Scholar] [CrossRef]
- Ray, C.; Lee, S.C.; Sankar, K.V.; Jin, B.; Lee, J.; Park, J.H.; Jun, S.C. Amorphous phosphorous-incorporated cobalt molybdenum sulfide on carbon cloth; efficient and stable electrocatalyst for enhanced overall water splitting over entire Ph values. ACS Appl. Mater. Interfaces 2017, 9, 37739–37749. [Google Scholar] [CrossRef]
- Dai, M.; Zhao, D.; Liu, H.; Tong, Y.; Hu, P.; Wu, X. Nanostructure and doping engineering of ZnCoP for high performance electrolysis of water. Mater. Today Eng. 2020, 16, 100412. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Asset, T.; Job, N.; Busby, Y.; Crisci, A.; Martin, V.; Stergiopoulos, V.; Bonnaud, C.; Serov, A.; Atanassov, P.; Chattot, R.; et al. Porous hollow PtNi/C electrocatalysts: Carbon support considerations to meet performance and stability requirements. ACS Catal. 2018, 8, 893–903. [Google Scholar] [CrossRef]
- Zhao, D.; Dai, M.; Zhao, Y.; Liu, H.; Liu, Y.; Wu, X. Improving electrocatalytic activities of FeCo2O4@FeCo2O4@PPy electrodes by surface/interface regulation. Nano Energy 2020, 72, 104715. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Y.; Zou, Y.; Zhang, N.; Zhang, Y.; Wu, Y.; Wang, Y.; Chen, R.; Wang, S. Charge Transfer Modulated Activity of Carbon-Based Electrocatalysts. Adv. Energy Mater. 2020, 10, 1901227. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Dai, M.; Liu, H.; Chen, K.; Zhu, X.; Xue, D.; Wu, X.; Liu, J. Sulfur-Induced Interface Engineering of Hybrid NiCo2O4@NiMo2S4 Structure for Overall Water Splitting and Flexible Hybrid Energy Storage. Adv. Mater. Interfaces 2019, 6, 1901308. [Google Scholar] [CrossRef]
- Shinde, N.; Shinde, P.; Xia, Q.X.; Yun, J.M.; Mane, R.; Kim, K.H. Electrocatalytic Water Splitting through the NixSy Self-Grown Superstructures Obtained via a Wet Chemical Sulfurization Process. ACS Omega 2019, 4, 6486–6491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Lu, X.; Zheng, G.; Ho, G.W. Topotactic engineering of ultrathin 2D nonlayered nickel selenides for full water electrolysis. Adv. Energy Mater. 2018, 8, 1702704. [Google Scholar] [CrossRef]
- Raut, S.D.; Mane, H.R.; Shinde, N.M.; Lee, D.; Shaikh, S.F.; Kim, K.H.; Kim, H.J.; Al-Enizi, A.M.; Mane, R.S. Electrochemically grown MnO2 nanowires for supercapacitor and electrocatalysis applications. New J. Chem. 2020, 44, 17864–17870. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Zhang, G.; Zhang, Z.; Fang, C.; Ma, H.; Luo, W.; Liu, Z. Enhanced Stability Lithium-Ion Battery Based on Optimized Graphene/Si Nanocomposites by Templated Assembly. ACS Omega 2019, 4, 18195–18202. [Google Scholar] [CrossRef]
- Patil, R.; Phadatare, M.; Blomquist, N.; Ortegren, J.; Hummelgard, M.; Meshram, J.; Dubal, D.; Olin, H. Highly Stable Cycling of Silicon-Nanographite Aerogel-BasedAnode for Lithium-Ion Batteries. ACS Omega 2021, 6, 6600–6606. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Park, S.; Hyeonju Lee, H.; Kim, H.; Chung, Y.-H.; Yoo, J.M.; Ahn, D.; Yu, S.-H.; Lee, K.-S.; Ahmadi, M.; et al. Activity−Stability Relationship in Au@Pt Nanoparticles for Electrocatalysis. ACS Energy Lett. 2020, 5, 2827–2834. [Google Scholar] [CrossRef]
- Muthurasu, A.; Dahal, B.; Mukhiya, T.; Chhetri, K.; Kim, H.Y. Fabrication of nonmetal-modulated dual metal-organic platform for overall water splitting and rechargeable zinc-air batteries. ACS Appl. Mater. Interfaces 2020, 12, 41704–41717. [Google Scholar] [CrossRef] [PubMed]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-W.; Anushya, G.; Chen, S.-M.; Kalimuthu, P.; Mariyappan, V.; Gajendran, P.; Ramachandran, R. Recent Advances in Nanoscale Based Electrocatalysts for Metal-Air Battery, Fuel Cell and Water-Splitting Applications: An Overview. Materials 2022, 15, 458. https://doi.org/10.3390/ma15020458
Chen T-W, Anushya G, Chen S-M, Kalimuthu P, Mariyappan V, Gajendran P, Ramachandran R. Recent Advances in Nanoscale Based Electrocatalysts for Metal-Air Battery, Fuel Cell and Water-Splitting Applications: An Overview. Materials. 2022; 15(2):458. https://doi.org/10.3390/ma15020458
Chicago/Turabian StyleChen, Tse-Wei, Ganesan Anushya, Shen-Ming Chen, Palraj Kalimuthu, Vinitha Mariyappan, Pandi Gajendran, and Rasu Ramachandran. 2022. "Recent Advances in Nanoscale Based Electrocatalysts for Metal-Air Battery, Fuel Cell and Water-Splitting Applications: An Overview" Materials 15, no. 2: 458. https://doi.org/10.3390/ma15020458