Modeling of the Human Bone Environment: Mechanical Stimuli Guide Mesenchymal Stem Cell–Extracellular Matrix Interactions
Abstract
:1. Introduction
2. Materials and Methods
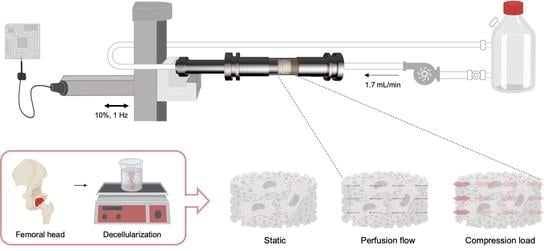
2.1. Preparation of Decellularized Bone Scaffolds
2.2. Elastic Modulus Measurements
2.3. Pore Size Measurements
2.4. Dynamic Bioreactor
2.5. Computational Fluid Dynamic
2.6. Mesenchymal Stem/Stromal Cells Isolation and Loading Protocol
2.7. Viability Assays
2.8. Immunofluorescence Analysis
2.9. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
2.10. Gene Transcription Analysis
2.11. Statistical Analysis
3. Results
3.1. Scaffold Structure Characterization
3.2. Computational Modeling
3.3. In Vitro Studies
3.3.1. hMSC—Scaffold Integration in the Static and Dynamic Culture
3.3.2. hMSC Early Response to Mechanical Stimuli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuchs, R.; Warden, S.; Turner, C. Bone anatomy, physiology and adaptation to mechanical loading. In Bone Repair Biomaterials; Elsevier: Sawston, UK, 2009; pp. 25–68. [Google Scholar]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The bone extracellular matrix in bone formation and regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Li, Y.; Aparicio, C. Discerning the subfibrillar structure of mineralized collagen fibrils: A model for the ultrastructure of bone. PLoS ONE 2013, 8, e76782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautieri, A.; Vesentini, S.; Redaelli, A.; Buehler, M.J. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett. 2011, 11, 757–766. [Google Scholar] [CrossRef]
- Fan, D.; Creemers, E.E.; Kassiri, Z. Matrix as an interstitial transport system. Circ. Res. 2014, 114, 889–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Alford, A.I.; Kozloff, K.M.; Hankenson, K.D. Extracellular matrix networks in bone remodeling. Int. J. Biochem. Cell Biol. 2015, 65, 20–31. [Google Scholar] [CrossRef]
- Pereira, A.; Trivanović, D.; Herrmann, M. Approaches to mimic the complexity of the skeletal mesenchymal stem/stromal cell niche in vitro. Eur. Cells Mater. 2019, 37, 88–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, E.H.; Klein-Nulend, J. Mechanotransduction in bone—Role of the lacunocanalicular network. FASEB J. 1999, 13, S101–S112. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ogawa, R. Mechanotransduction in bone repair and regeneration. FASEB J. 2010, 24, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Grellier, M.; Bareille, R.; Bourget, C.; Amédée, J. Responsiveness of human bone marrow stromal cells to shear stress. J. Tissue Eng. Regen. Med. 2009, 3, 302–309. [Google Scholar] [CrossRef]
- Yourek, G.; McCormick, S.M.; Mao, J.J.; Reilly, G.C. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen. Med. 2010, 5, 713–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.A.; Chatterjee-Kishore, M.; Yaworsky, P.J.; Cullen, D.M.; Zhao, W.; Li, C.; Kharode, Y.; Sauter, L.; Babij, P.; Brown, E.L. Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 2006, 281, 31720–31728. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sittichockechaiwut, A.; Scutt, A.M.; Ryan, A.J.; Bonewald, L.F.; Reilly, G.C. Use of rapidly mineralising osteoblasts and short periods of mechanical loading to accelerate matrix maturation in 3D scaffolds. Bone 2009, 44, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, A.; Lim, J.; Chong, M.S.K.; Wen, F.; Liu, Y.; Pillay, Y.T.; Chan, J.K.; Teoh, S.H. In vitro cyclic compressive loads potentiate early osteogenic events in engineered bone tissue. J. Biomed. Mater. Res. Appl. Biomater. 2017, 105, 2366–2375. [Google Scholar] [CrossRef]
- Hsieh, Y.F.; Turner, C.H. Effects of loading frequency on mechanically induced bone formation. J. Bone Miner. Res. 2001, 16, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Geris, L.; Guyot, Y.; Schrooten, J.; Papantoniou, I. In silico regenerative medicine: How computational tools allow regulatory and financial challenges to be addressed in a volatile market. Interface Focus 2016, 6, 20150105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgi, M.; Verbruggen, S.W.; Lacroix, D. In silico bone mechanobiology: Modeling a multifaceted biological system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 485–505. [Google Scholar] [CrossRef] [Green Version]
- Muschler, G.F.; Raut, V.P.; Patterson, T.E.; Wenke, J.C.; Hollinger, J.O. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng. Rev. 2010, 16, 123–145. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.S. Animal models of osteoporosis—Necessity and limitations. Eur. Cell Mater 2001, 1, 13. [Google Scholar]
- Zeiter, S.; Koschitzki, K.; Alini, M.; Jakob, F.; Rudert, M.; Herrmann, M. Evaluation of preclinical models for the testing of bone tissue-engineered constructs. Tissue Eng. Methods 2020, 26, 107–117. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Nuss, K.M.; von Rechenberg, B. Biocompatibility issues with modern implants in bone—A review for clinical orthopedics. Open Orthop. J. 2008, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.M.; Haugh, M.G.; O’brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen—Glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Rothrauff, B.B.; Tuan, R.S. Decellularized bone extracellular matrix in skeletal tissue engineering. Biochem. Soc. Trans. 2020, BST20190079. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Zhang, M.; Powers, R.M.; Wolfinbarger, L. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J. Periodontol. 1997, 68, 1085–1092. [Google Scholar] [CrossRef]
- Pereira, A.R.; Rudert, M.; Herrmann, M. Decellularized human bone as a 3D model to study skeletal progenitor cells in a natural environment. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 157, pp. 123–141. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Ramani-Mohan, R.K.; Schwedhelm, I.; Finne-Wistrand, A.; Krug, M.; Schwarz, T.; Jakob, F.; Walles, H.; Hansmann, J. Deformation strain is the main physical driver for skeletal precursors to undergo osteogenesis in earlier stages of osteogenic cell maturation. J. Tissue Eng. Regen. Med. 2018, 12, e1474–e1479. [Google Scholar] [CrossRef]
- Phinney, D.G.; Kopen, G.; Righter, W.; Webster, S.; Tremain, N.; Prockop, D.J. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J. Cell. Biochem. 1999, 75, 424–436. [Google Scholar] [CrossRef]
- Rady, D.; Abbass, M.; El-Rashidy, A.A.; El Moshy, S.; Radwan, I.A.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Mesenchymal stem/progenitor cells: The prospect of human clinical translation. Stem Cells Int. 2020, 2020, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Jakob, F. Bone marrow niches for skeletal progenitor cells and their inhabitants in health and disease. Curr. Stem Cell Res. Ther. 2019, 14, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, R.U.; Kemper, N.; Breathwaite, E.; Dutta, S.M.; Huber, A.; Murchison, A.; Chen, S.; Hsu, E.L.; Hsu, W.K.; Francis, M.P. Demineralized bone matrix fibers formable as general and custom 3D printed mold-based implants for promoting bone regeneration. Biofabrication 2016, 8, 035007. [Google Scholar] [CrossRef]
- Smith, C.A.; Board, T.N.; Rooney, P.; Eagle, M.J.; Richardson, S.M.; Hoyland, J.A. Human decellularized bone scaffolds from aged donors show improved osteoinductive capacity compared to young donor bone. PLoS ONE 2017, 12, e0177416. [Google Scholar] [CrossRef] [Green Version]
- Bianco, J.E.R.; Rosa, R.G.; Congrains-Castillo, A.; Joazeiro, P.P.; Waldman, S.D.; Weber, J.F.; Saad, S.T.O. Characterization of a novel decellularized bone marrow scaffold as an inductive environment for hematopoietic stem cells. Biomater. Sci. 2019, 7, 1516–1528. [Google Scholar] [CrossRef]
- Rubert, M.; Vetsch, J.R.; Lehtoviita, I.; Sommer, M.; Zhao, F.; Studart, A.R.; Müller, R.; Hofmann, S. Scaffold pore geometry influences bone-like tissue formation in dynamic cell culture conditions. BioRxiv 2020. [Google Scholar]
- Van Tol, A.F.; Schemenz, V.; Wagermaier, W.; Roschger, A.; Razi, H.; Vitienes, I.; Fratzl, P.; Willie, B.M.; Weinkamer, R. The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. Proc. Natl. Acad. Sci. USA 2020, 117, 32251–32259. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Shoumura, S.; Emura, S.; Bunai, Y. Age-and gender-dependent changes in three-dimensional microstructure of cortical and trabecular bone at the human femoral neck. Osteoporos. Int. 2010, 21, 627–636. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.I.; Wang, X.; Wu, T.J.; Lee, Y.T.; Kim, S.; Miguez, P.; Ko, C.C. Effect of pore size in bone regeneration using polydopamine-laced hydroxyapatite collagen calcium silicate scaffolds fabricated by 3D mould printing technology. Orthod. Craniofacial Res. 2019, 22, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbühl, R.; Bohner, M.; Richter, W. Porosity and pore size of β-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Campos, I.; Marolt, D.; Petridis, P.; Bhumiratana, S.; Schmidt, D.; Vunjak-Novakovic, G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials 2012, 33, 8329–8342. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Xue, R.; Li, J.Y.S.; Yeh, Y.; Yang, L.; Chien, S. Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation. J. Orthop. Res. 2013, 31, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.H.; Chan, H.L.; Chong, L.Y.; Jheng, Y.H.; Chang, P.C. Evaluation of the osteogenic potential of growth factor—Rich demineralized bone matrix in vivo. J. Periodontol. 2015, 86, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Wang, J.; Qiu, P.; Wang, S.; Shi, Y.; Li, M.; Chen, P.; Lin, X.; Fang, X. A cancellous bone matrix system with specific mineralisation degrees for mesenchymal stem cell differentiation and bone regeneration. Biomater. Sci. 2019, 7, 2452–2467. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Rodríguez, G.B.; Montesi, M.; Panseri, S.; Sprio, S.; Tampieri, A.; Sandri, M. Biomineralized recombinant collagen-based scaffold mimicking native bone enhances mesenchymal stem cell interaction and differentiation. Tissue Eng. 2017, 23, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.B.; Pereira, A.R.; Herrmann, M.; Hansmann, J.; Delgado-López, J.M.; Sprio, S.; Tampieri, A.; Sandri, M. Biomimetic mineralization promotes viability and differentiation of human mesenchymal stem cells in a perfusion bioreactor. Int. J. Mol. Sci. 2021, 22, 1447. [Google Scholar] [CrossRef]
- Becquart, P.; Cruel, M.; Hoc, T.; Sudre, L.; Pernelle, K.; Bizios, R.; Logeart-Avramoglou, D.; Petite, H.; Bensidhoum, M. Human mesenchymal stem cell responses to hydrostatic pressure and shear stress. Eur. Cell Mater. 2016, 31, 160–173. [Google Scholar] [CrossRef]
- Metzger, T.A.; Schwaner, S.A.; LaNeve, A.J.; Kreipke, T.C.; Niebur, G.L. Pressure and shear stress in trabecular bone marrow during whole bone loading. J. Biomech. 2015, 48, 3035–3043. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Zhang, Y.; Jing, D.; Shen, Y.; Tang, G.; Huang, S.; Zhao, Z. Mechanobiology of mesenchymal stem cells: Perspective into mechanical induction of MSC fate. Acta Biomater. 2015, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Srinivasan, A.; Leung, C.M.; Toh, Y.-C. Bio-mimicking shear stress environments for enhancing mesenchymal stem cell differentiation. Curr. Stem Cell Res. Ther. 2020, 15, 414–427. [Google Scholar] [CrossRef]
- Melke, J.; Zhao, F.; van Rietbergen, B.; Ito, K.; Hofmann, S. Localisation of mineralised tissue in a complex spinner flask environment correlates with predicted wall shear stress level localisation. Eur. Cells Mater. 2018, 36, 57–68. [Google Scholar] [CrossRef]
- Yamada, M.A.Y.; Schwarz, T.; Hansmann, J.; Mustafa, K. Induction of osteogenic differentiation of bone marrow stromal cells on 3D polyester-based scaffolds solely by subphysiological fluidic stimulation in a laminar flow bioreactor. J. Tissue Eng. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Palmroth, A.; Pitkänen, S.; Hannula, M.; Paakinaho, K.; Hyttinen, J.; Miettinen, S.; Kellomäki, M. Evaluation of scaffold microstructure and comparison of cell seeding methods using micro-computed tomography-based tools. J. R. Soc. Interface 2020, 17, 20200102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef]
- De Girolamo, L.; Sartori, M.; Arrigoni, E.; Rimondini, L.; Albisetti, W.; Weinstein, R.; Brini, A.T. Human adipose-derived stem cells as future tools in tissue regeneration: Osteogenic differentiation and cell-scaffold interaction. Int. J. Artif. Organs 2008, 31, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Sittichokechaiwut, A.; Edwards, J.; Scutt, A.; Reilly, G. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cell. Eur. Cells Mater. 2010, 20, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Morris, H.; Reed, C.; Haycock, J.; Reilly, G. Mechanisms of fluid-flow-induced matrix production in bone tissue engineering. Proc. Inst. Mech. Eng. J. Eng. Med. 2010, 224, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.K.; Puellen, A.; Kramann, R.; Raupach, K.; Bornemann, J.; Knuechel, R.; Pérez-Bouza, A.; Neuss, S. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials 2010, 31, 467–480. [Google Scholar] [CrossRef]
- Toosi, S.; Naderi-Meshkin, H.; Kalalinia, F.; Peivandi, M.T.; HosseinKhani, H.; Bahrami, A.R.; Heirani-Tabasi, A.; Mirahmadi, M.; Behravan, J. PGA-incorporated collagen: Toward a biodegradable composite scaffold for bone-tissue engineering. J. Biomed. Mater. Res. 2016, 104, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; Gao, Q.; Kotecha, M.; Magin, R.L.; Karol, S.; Bedran-Russo, A.; George, A. Biomimetic extracellular matrix-incorporated scaffold induces osteogenic gene expression in human marrow stromal cells. Tissue Eng. 2012, 18, 295–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elango, J.; Robinson, J.; Zhang, J.; Bao, B.; Ma, N.; de Val, J.E.M.S.; Wu, W. Collagen peptide upregulates osteoblastogenesis from bone marrow mesenchymal stem cells through MAPK-Runx2. Cells 2019, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Bautista, D.S.; Xuan, J.; Hota, C.; Chambers, A.F.; Harris, J.F. Inhibition of Arg-Gly-Asp (RGD)-mediated cell adhesion to osteopontin by a monoclonal antibody against osteopontin. J. Biol. Chem. 1994, 269, 23280–23285. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zhang, L.; Xu, C.; Zheng, C.; Han, Y.; Li, W.; Huang, Y.; Zhang, X.; Shao, C. An osteopontin-integrin interaction plays a critical role in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem Cells 2014, 32, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Zappone, B.; Thurner, P.J.; Adams, J.; Fantner, G.E.; Hansma, P.K. Effect of Ca2+ ions on the adhesion and mechanical properties of adsorbed layers of human osteopontin. Biophys. J. 2008, 95, 2939–2950. [Google Scholar] [CrossRef] [Green Version]
- Terai, K.; Takano-Yamamoto, T.; Ohba, Y.; Hiura, K.; Sugimoto, M.; Sato, M.; Kawahata, H.; Inaguma, N.; Kitamura, Y.; Nomura, S. Role of osteopontin in bone remodeling caused by mechanical stress. J. Bone Miner. Res. 1999, 14, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Morinobu, M.; Ishijima, M.; Rittling, S.R.; Tsuji, K.; Yamamoto, H.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J. Bone Miner. Res. 2003, 18, 1706–1715. [Google Scholar] [CrossRef]
- Darnell, M. Mechanotransduction Across Time and Length Scales. PhD Thesis, Harvard University, Cambridge, MA, USA, 2017. [Google Scholar]
- Dalby, M.J. Topographically induced direct cell mechanotransduction. Med. Eng. Phys. 2005, 27, 730–742. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckman, C.A.; Plummer, H. Filopodia as sensors. Cell. Signal. 2013, 25, 2298–2311. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.J.; Jacobs, C.R. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res. Embryo Today Rev. 2010, 90, 75–85. [Google Scholar] [CrossRef]
- Peake, M.; Cooling, L.; Magnay, J.; Thomas, P.; El Haj, A. Selected contribution: Regulatory pathways involved in mechanical induction of c-fos gene expression in bone cells. J. Appl. Physiol. 2000. [Google Scholar] [CrossRef] [Green Version]
- Joldersma, M.; Burger, E.H.; Semeins, C.M.; Klein-Nulend, J. Mechanical stress induces COX-2 mRNA expression in bone cells from elderly women. J. Biomech. 2000, 33, 53–61. [Google Scholar] [CrossRef]
- Müller-Deubert, S.; Seefried, L.; Krug, M.; Jakob, F.; Ebert, R. Epidermal growth factor as a mechanosensitizer in human bone marrow stromal cells. Stem Cell Res. 2017, 24, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Friedl, G.; Schmidt, H.; Rehak, I.; Kostner, G.; Schauenstein, K.; Windhager, R. Undifferentiated human mesenchymal stem cells (hMSCs) are highly sensitive to mechanical strain: Transcriptionally controlled early osteo-chondrogenic response in vitro. Osteoarthr. Cartil. 2007, 15, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. 2017, 35, 2378–2385. [Google Scholar] [CrossRef]
- Gilbert, M.; Shaw, W.J.; Long, J.R.; Nelson, K.; Drobny, G.P.; Giachelli, C.M.; Stayton, P.S. Chimeric peptides of statherin and osteopontin that bind hydroxyapatite and mediate cell adhesion. J. Biol. Chem. 2000, 275, 16213–16218. [Google Scholar] [CrossRef] [Green Version]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in bone metabolism and bone diseases. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919159-1–e919159-9. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Roelofsen, J.; Semeins, C.M.; Bronckers, A.L.; Burger, E.H. Mechanical stimulation of osteopontin mRNA expression and synthesis in bone cell cultures. J. Cell. Physiol. 1997, 170, 174–181. [Google Scholar] [CrossRef]
- Ishijima, M.; Tsuji, K.; Rittling, S.R.; Yamashita, T.; Kurosawa, H.; Denhardt, D.T.; Nifuji, A.; Ezura, Y.; Noda, M. Osteopontin is required for mechanical stress-dependent signals to bone marrow cells. J. Endocrinol. 2007, 193, 235–243. [Google Scholar] [CrossRef] [Green Version]
- McNeill, E.P.; Zeitouni, S.; Pan, S.; Haskell, A.; Cesarek, M.; Tahan, D.; Clough, B.H.; Krause, U.; Dobson, L.K.; Garcia, M. Characterization of a pluripotent stem cell-derived matrix with powerful osteoregenerative capabilities. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, E.; Reinboth, B.; Gibson, M.A. Covalent and non-covalent interactions of βig-h3 with collagen VI: βig-h3 is covalently attached to the amino-terminal region of collagen VI in tissue microfibrils. J. Biol. Chem. 2003, 278, 24334–24341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clough, B.H.; McCarley, M.R.; Krause, U.; Zeitouni, S.; Froese, J.J.; McNeill, E.P.; Chaput, C.D.; Sampson, H.W.; Gregory, C.A. Bone regeneration with osteogenically enhanced mesenchymal stem cells and their extracellular matrix proteins. J. Bone Miner. Res. 2015, 30, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Izu, Y.; Ezura, Y.; Koch, M.; Birk, D.E.; Noda, M. Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis. Cell Tissue Res. 2016, 364, 623–635. [Google Scholar] [CrossRef] [Green Version]
- Toma, C.; Ashkar, S.; Gray, M.; Schaffer, J.; Gerstenfeld, L. Signal transduction of mechanical stimuli is dependent on microfilament integrity: Identification of osteopontin as a mechanically induced gene in osteoblasts. J. Bone Miner. Res. 1997, 12, 1626–1636. [Google Scholar] [CrossRef]
- Hu, D.D.; Lin, E.C.; Kovach, N.L.; Hoyer, J.R.; Smith, J.W. A biochemical characterization of the binding of osteopontin to integrins αvβ1 and αvβ5. J. Biol. Chem. 1995, 270, 26232–26238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; McNamara, L.E.; Gadegaard, N.; Alakpa, E.V.; Burgess, K.V.; Meek, R.D.; Dalby, M.J. Nanotopographical induction of osteogenesis through adhesion, bone morphogenic protein cosignaling, and regulation of microRNAs. ACS Nano 2014, 8, 9941–9953. [Google Scholar] [CrossRef]
- Rui, Y.F.; Lui, P.P.Y.; Lee, Y.W.; Chan, K.M. Higher BMP receptor expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared with bone-marrow-derived mesenchymal stem cells. Int. Orthop. 2012, 36, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- Sumanasinghe, R.D.; Bernacki, S.H.; Loboa, E.G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: Effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006, 12, 3459–3465. [Google Scholar] [CrossRef]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Wulsten, D.; Glatt, V.; Ellinghaus, A.; Schmidt-Bleek, K.; Petersen, A.; Schell, H.; Lienau, J.; Sebald, W.; Plöger, F.; Seemann, P. Time kinetics of bone defect healing in response to BMP-2 and GDF-5 characterised by in vivo biomechanics. Eur. Cell Mater. 2011, 21, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, I.; Hisanaga, R.; Sato, T.; Arano, T.; Nomoto, S.; Ikada, Y.; Yoshinari, M. Effect of osteogenic differentiation medium on proliferation and differentiation of human mesenchymal stem cells in three-dimensional culture with radial flow bioreactor. Regen. Ther. 2015, 2, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Schreivogel, S.; Kuchibhotla, V.; Knaus, P.; Duda, G.N.; Petersen, A. Load-induced osteogenic differentiation of mesenchymal stromal cells is caused by mechano-regulated autocrine signaling. J. Tissue Eng. Regen. Med. 2019, 13, 1992–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardingham, T.E.; Oldershaw, R.A.; Tew, S.R. Cartilage, SOX9 and Notch signals in chondrogenesis. J. Anat. 2006, 209, 469–480. [Google Scholar] [CrossRef]
- Kupcsik, L.; Stoddart, M.J.; Li, Z.; Benneker, L.M.; Alini, M. Improving chondrogenesis: Potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng. 2010, 16, 1845–1855. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.R.; Lipphaus, A.; Ergin, M.; Salehi, S.; Gehweiler, D.; Rudert, M.; Hansmann, J.; Herrmann, M. Modeling of the Human Bone Environment: Mechanical Stimuli Guide Mesenchymal Stem Cell–Extracellular Matrix Interactions. Materials 2021, 14, 4431. https://doi.org/10.3390/ma14164431
Pereira AR, Lipphaus A, Ergin M, Salehi S, Gehweiler D, Rudert M, Hansmann J, Herrmann M. Modeling of the Human Bone Environment: Mechanical Stimuli Guide Mesenchymal Stem Cell–Extracellular Matrix Interactions. Materials. 2021; 14(16):4431. https://doi.org/10.3390/ma14164431
Chicago/Turabian StylePereira, Ana Rita, Andreas Lipphaus, Mert Ergin, Sahar Salehi, Dominic Gehweiler, Maximilian Rudert, Jan Hansmann, and Marietta Herrmann. 2021. "Modeling of the Human Bone Environment: Mechanical Stimuli Guide Mesenchymal Stem Cell–Extracellular Matrix Interactions" Materials 14, no. 16: 4431. https://doi.org/10.3390/ma14164431