Properties and Applications of Nanoparticles from Plant Proteins
Abstract
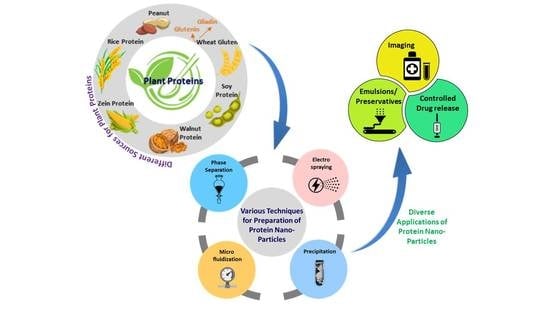
:1. Introduction
2. Nanoparticles from Zein
2.1. Preparation and Stabilization of Zein Nanoparticles
2.2. Zein Nanoparticles for Controlled Release Applications
2.3. Curcumin Delivery through Zein Nanoparticles
2.4. Controlled Release of Drugs Using Zein Nanoparticles
2.5. Zein Nanoparticles for Imaging
2.6. Nanocapsules and Core-Shell Zein Nanoparticles
3. Nanoparticles from Wheat Proteins
3.1. Gliadin Nanoparticles
Gliadin Nanoparticles for Food Applications
3.2. Nanoparticles from Wheat Glutenin
4. Nanoparticles from Soy Proteins
4.1. Soy Protein Nanoparticles for Controlled Release Applications
4.2. Applications of Soy Protein Nanoparticles in the Food Industry
5. Nanoparticles from Miscellaneous Plant Proteins
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yetisgin, A.A.; Sibel, C.; Merve, Z.; Ali, K.; Ozlem, K. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Alsaba, M.T.; Mohammed, F.A.D.; Ahmed, K.A. A comprehensive review of nanoparticles applications in the oil and gas industry. J. Petr. Expl. Prod. Technol. 2020, 10, 1389–1399. [Google Scholar] [CrossRef] [Green Version]
- Braeken, Y.; Srujan, C.; Anitha, E.; Wouter, M. Conjugated polymer nanoparticles for bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, I.; Jong-Beom, B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. In Natural Polymer Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2016; pp. 33–93. [Google Scholar]
- Atanase, L.I. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Liying, W.; Yi, C.C.; Yon, R. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Sumit, K.S.; Shailendra, K.A.; Subhas, C.K.; Sonia, K. Protein nanoparticles: Promising platforms for drug delivery applications. ACS Biomat. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011, 29, 490–498. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein-based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Jan, A.D. Cereal protein-based nanoparticles as agents stabilizing air–water and oil–water interfaces in food systems. Curr. Opin. Food Sci. 2019, 25, 19–27. [Google Scholar] [CrossRef]
- Sağlam, D.; Paul, V.; Erik, L.; Renko, V. Design, properties, and applications of protein micro-and nanoparticles. Curr. Opin. Coll. Inter. Sci. 2014, 19, 428–437. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Mari Pau, B.; Rafael, G.; Pilar, H. Formation of zein nanoparticles by electrohydrodynamic atomization: Effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocoll. 2012, 28, 82–91. [Google Scholar] [CrossRef]
- Chen, H.; Qixin, Z. Processes improving the dispersibility of spray-dried zein nanoparticles using sodium caseinate. Food Hydrocoll. 2014, 35, 358–366. [Google Scholar] [CrossRef]
- Wang, D.; Shengnan, T.; Shou-Wei, Y.; Yajuan, S.; Yunxing, L. Facile preparation of zein nanoparticles with tunable surface hydrophobicity and excellent colloidal stability. Coll. Surf. A Physico. Eng. Asp. 2020, 591, 124554–124562. [Google Scholar] [CrossRef]
- Feng, X.; Yujia, S.; Yuyan, Y.; Xin, Z.; Kaiyue, C.; Chen, Y.; Tian, X.; Xiaozhi, T. Zein nanoparticle stabilized Pickering emulsion enriched with cinnamon oil and its effects on pound cakes. LWT 2020, 122, 109025–109032. [Google Scholar] [CrossRef]
- Zhong, Q.; Minfeng, J. Zein nanoparticles produced by liquid–liquid dispersion. Food Hydrocoll. 2009, 23, 2380–2387. [Google Scholar] [CrossRef]
- Luis, A.I.S.; Estefania, V.R.C.; Jhones, L.D.O.; Mariana, G.; Renata, D.L.; Rodrigo, F.C.; Vera, L.S.S.D.; Fraceto, L.F. Zein Nanoparticles Impregnated with Eugenol and Garlic Essential Oils for Treating Fish Pathogens. ACS Omega 2020, 5, 15557–15566. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. A novel method of preparing stable zein nanoparticle dispersions for encapsulation of peppermint oil. Food Hydrocoll. 2015, 43, 593–602. [Google Scholar] [CrossRef]
- Pascoli, M.; Renata, D.; Leonardo, F.F. Zein nanoparticles and strategies to improve colloidal stability: A mini-review. Front. Chem. 2018, 6, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Hernández, J.; Francisco, R.; Josué, E.; Saúl, R.; Miguel, A.R.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef]
- Soltani, S.; Madadlou, A. Gelation characteristics of the sugar beet pectin solution charged with fish oil-loaded zein nanoparticles. Food Hydrocoll. 2015, 43, 664–669. [Google Scholar] [CrossRef]
- Dai, L.; Cuixia, S.; Ruirui, L.; Like, M.; Fuguo, L.; Yanxiang, G. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dai, Y.; Cai, J.; Zhong, N.; Xiao, H.; McClements, D.J.; Hu, K. Resveratrol encapsulation in core-shell biopolymer nanoparticles: Impact on antioxidant and anticancer activities. Food Hydrocoll. 2017, 64, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Caseinate-zein-polysaccharide complex nanoparticles as potential oral delivery vehicles for curcumin: Effect of polysaccharide type and chemical cross-linking. Food Hydrocoll. 2017, 72, 254–262. [Google Scholar] [CrossRef]
- Veneranda, M.; Hu, Q.; Wang, T.; Luo, Y.; Castro, K.; Madariaga, J.M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT 2018, 89, 596–603. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Zhou, M.; Xue, J.; Luo, Y. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for curcumin. Food Hydrocoll. 2017, 70, 143–151. [Google Scholar] [CrossRef]
- Huang, X.; Huang, X.; Gong, Y.; Xiao, H.; McClements, D.J.; Hu, K. Enhancement of curcumin water dispersibility and antioxidant activity using core –shell protein–polysaccharide nanoparticles. Food Res. Int. 2016, 87, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Teng, Z.; Wang, Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J. Agric. Food Chem. 2012, 60, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, T.T.; Teng, Z.; Chen, P.; Sun, J.; Wang, Q. Encapsulation of indole-3-carbinol and 3, 3′-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. 2013, 139, 224–230. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- Wang, M.; Yuying, F.; Guowen, C.; Yugang, S.; Xiaomeng, L.; Hao, Z.; Yali, S. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Liang, H.; Huang, Q.; Zhou, B.; He, L.; Lin, L.; An, Y.; Li, B. Self-assembled zein–sodium carboxymethyl cellulose nanoparticles as an effective drug carrier and transporter. J. Mater. Chem. B 2015, 3, 3242–3253. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Joye, I.J.; McClements, D.J. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 1: Preparation and characterization. Food Hydrocoll. 2015, 45, 309–316. [Google Scholar] [CrossRef]
- Sun, C.; Xu, C.; Mao, L.; Wang, D.; Yang, J.; Gao, Y. Preparation, characterization and stability of curcumin-loaded zein-shellac composite colloidal particles. Food Chem. 2017, 228, 656–667. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, B.; He, L.; An, Y.; Lin, L.; Li, Y.; Li, B. Fabrication of zein/quaternized chitosan nanoparticles for the encapsulation and protection of curcumin. RSC Adv. 2015, 5, 13891–13900. [Google Scholar] [CrossRef]
- Liang, H.; Bin, Z.; Jing, L.; Wei, X.; Shilin, L.; Yan, L.; Yijie, C.; Bin, L. Supramolecular design of coordination bonding architecture on zein nanoparticles for pH-responsive anticancer drug delivery. Coll. Surf. B Bioint. 2015, 136, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.T.; Hsieh, S.Y.; Chen, T.H.; Hung, P.F.; Pan, S.H. Sorafenib- fortified zein–chondroitin sulphate biopolymer nanoparticles as a novel therapeutic system in gastric cancer treatment. RSC Adv. 2016, 6, 57266–57274. [Google Scholar] [CrossRef]
- Lai, L.F.; Guo, H.X. Preparation of new 5-fluorouracilloaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, Y.; Luo, Y.; Ge, M.; Yang, T.; Yu, L.; Wang, Q. Fabrication, characterization and antimicrobial activities of thymol-loaded zein nanoparticles stabilized by sodium caseinate–chitosan hydrochloride double layers. Food Chem. 2014, 142, 269–275. [Google Scholar] [CrossRef]
- da Rosa, C.G.; de Oliveira, B.M.; de Carvalho, M.V.; de Melo, S.M.; Jummes, B.; Da Silva, T.; Martelli, S.M.; Viletti, M.A.; Bertoldi, F.C.; Baretto, P.L.M. Characterization and evaluation of physicochemical and antimicrobial properties of zein nanoparticles loaded with phenolics monoterpenes. Colloids Surf. Physicochem. Eng. Asp. 2015, 481, 337–344. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. Physicochem. Eng. Asp. 2016, 503, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Dai, L.; He, X.; Liu, F.; Yuan, F.; Gao, Y. Effect of heat treatment on physical, structural, thermal and morphological characteristics of zein in ethanol-water solution. Food Hydrocoll. 2016, 58, 11–19. [Google Scholar] [CrossRef]
- Cheng, C.J.; Jones, O.G. Stabilizing zein nanoparticle dispersions with -carrageenan. Food Hydrocoll. 2017, 69, 28–35. [Google Scholar] [CrossRef]
- Pauluk, D.; Krause Padilha, A.; Khalil, N.M.; Mainardes, R.M. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocoll. 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Yuan, Y.; Hao, L.; Chengzhen, L.; Junxiang, Z.; Ying, X.; Shuaizhong, Z.; Minghao, F.; Zhang, D.; Zhang, Y.; Zhang, Z.; et al. Fabrication of stable zein nanoparticles by chondroitin sulfate deposition based on antisolvent precipitation method. Int. J. Biol. Macromol. 2019, 139, 30–39. [Google Scholar] [CrossRef]
- de Araujo, J.T.C.; Martin-Pastor, M.; Pérez, L.; Pinazo, A.; de Sousa, F.F.O. Development of anacardic acid-loaded zein nanoparticles: Physical chemical characterization, stability and antimicrobial improvement. J. Mol. Liq. 2021, 332, 115808. [Google Scholar] [CrossRef]
- Yao, K.; Chen, W.; Song, F.; McClements, D.J.; Hu, K. Tailoring zein nanoparticle functionality using biopolymer coatings: Impact on curcumin bioaccessibility and antioxidant capacity under simulated gastrointestinal conditions. Food Hydrocoll. 2018, 79, 262–272. [Google Scholar] [CrossRef]
- Menghua, L.; Fang, S.; Zhao, X.; Liang, X.; Wu, D. Natamycin-loaded zein nanoparticles stabilized by carboxymethyl chitosan: Evaluation of colloidal/chemical performance and application in postharvest treatments. Food Hydrocoll. 2020, 106, 105871. [Google Scholar]
- Zou, T.; Zheng, L.; Susan, S.P.; Suzanna, B.; Liwei, G. Fabrication, characterization, and cytotoxicity evaluation of cranberry procyanidins-zein nanoparticles. Food Hydrocoll. 2012, 27, 293–300. [Google Scholar] [CrossRef]
- Calliari, C.M.; Roberta, C.; Margherita, P.; Patrizia, P. Encapsulation of Hibiscus sabdariffa extract into zein nanoparticles. Chem. Eng. Technol. 2020, 43, 2062–2072. [Google Scholar] [CrossRef]
- Gagliardi, A.; Donatella, P.; Nicola, C.; Massimo, F.; Donato, C. Zein-vs PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C 2021, 118, 111538–111544. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Donatella, P.; Michelangelo, I.; Ernesto, P.; Massimo, F.; Donato, C. Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system. Int. J. Nanomed. 2018, 13, 601–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Dong-June, P.; Bum-Keun, K. Effects of a chitosan coating on properties of retinol-encapsulated zein nanoparticles. Food Sci. Biotechnol. 2015, 24, 1725–1733. [Google Scholar] [CrossRef]
- Zhang, H.; Yuying, F.; Fuge, N.; Zeya, L.; Chujie, B.; Bing, J.; Guowen, C.; Xiaomeng, L. Enhanced antioxidant activity and in vitro release of propolis by acid-induced aggregation using heat-denatured zein and carboxymethyl chitosan. Food Hydrocoll. 2018, 81, 104–112. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Cristina, M.S. Zein nanoparticles as delivery systems for covalently linked and physically entrapped folic acid. J. Nanopart. Res. 2017, 19, 81–93. [Google Scholar] [CrossRef]
- Brotons-Canto, A.; Carlos, J.G.; Ana Gloria Gil, E.A.; María, J.S.; Josep, M.L. Zein Nanoparticles Improve the Oral Bioavailability of Curcumin in Wistar Rats. Pharmaceutics 2021, 13, 361. [Google Scholar] [CrossRef]
- Yu, Y.; Ming-Yu, W.; Chun, W.; Zi-Wei, W.; Ting-Ting, C.; Jing-Kun, Y. Constructing biocompatible carboxylic curdlan-coated zein nanoparticles for curcumin encapsulation. Food Hydrocoll. 2020, 108, 106028. [Google Scholar] [CrossRef]
- Yuan, Y.; Hao, L.; Junxiang, Z.; Chengzhen, L.; Xun, S.; Dongfeng, W.; Ying, X. Fabrication and characterization of zein nanoparticles by dextran sulfate coating as vehicles for delivery of curcumin. Int. J. Biol. Macromol. 2020, 151, 1074–1083. [Google Scholar] [CrossRef]
- Sun, X.; Chuqiao, P.; Zhuoyang, Y.; Dongyan, Y.; Xitai, D.; Fangfang, H.; Junhong, L.; Xiao-kun, Q. Stabilization of zein nanoparticles with k-carrageenan and tween 80 for encapsulation of curcumin. Int. J. Biol. Macromol. 2020, 146, 549–559. [Google Scholar]
- Yu, X.; Huichao, W.; Haiyan, H.; Ziyi, D.; Yunni, D.; Qi, Q.; Yan, W.; Shouying, D.; Yang, L. Zein nanoparticles as nontoxic delivery system for maytansine in the treatment of non-small cell lung cancer. Drug Deliv. 2020, 27, 100–109. [Google Scholar]
- Liu, Q.; Jingjing, C.; Yang, Q.; Bo, J.; Tao, Z. Zein/fucoidan-based composite nanoparticles for the encapsulation of pterostilbene: Preparation, characterization, physicochemical stability, and formation mechanism. Int. J. Biol. Macromol. 2020, 158, 461–470. [Google Scholar]
- Nunes, R.; Ana, B.; Diana, M.; José, N.; Bruno, S. Zein nanoparticles as low-cost, safe, and effective carriers to improve the oral bioavailability of resveratrol. Drug Deliv. Trans. Res. 2020, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Nae-Won, K.; Hyelim, K.; Dong, H.; Jung-woo, C.; Lee, W.; Song, G.Y.; Cho, C.W.; Kim, D.D.; Lee, J.Y. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carb. Polym. 2021, 253, 117187–117194. [Google Scholar]
- Feng, S.; Yuxin, S.; Dan, W.; Peilong, S.; Ping, S. Effect of adjusting pH and chondroitin sulfate on the formation of curcumin-zein nanoparticles: Synthesis, characterization and morphology. Carb. Polym. 2020, 250, 116970–116982. [Google Scholar]
- Regier, M.C.; Taylor, J.D.; Borcyk, T.; Yang, Y.; Pannier, A.K. Fabrication and characterization of DNA-loaded zein nanospheres. J. Nanobiotechnol. 2012, 10, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aswathy, R.G.; Balasubramanian, S.; Dhandayudhapani, B.; Takahiro, F.; Yasuhiko, Y.; Toru, M.; Sakthi Kumar, D. Biocompatible fluorescent zein nanoparticles for simultaneous bioimaging and drug delivery application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025006. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Arunkumar, P.; Rajendra, P.; Sumit, K.M.; Pradeep, B.; Reddy, L.; Abhijit, D.; Rohit, S. Facile synthesis of plasmonic zein nanoshells for imaging-guided photothermal cancer therapy. Mater. Sci. Eng. C. 2018, 90, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, Q.; Reddy, N.; Yang, Y. Hollow nanoparticles from zein for potential medical applications. J. Mater. Chem. 2011, 21, 18227–18235. [Google Scholar]
- Xu, H.; Zhang, Y.; Jiang, Q.; Reddy, N.; Yang, Y. Biodegradable hollow zein nanoparticles for removal of reactive dyes from wastewater. J. Environ. Manag. 2013, 125, 33–40. [Google Scholar]
- Hu, K.; McClements, J.M. Fabrication of surfactant-stabilized zein nanoparticles: A pH modulated antisolvent precipitation method. Food Res. Int. 2014, 64, 329–335. [Google Scholar]
- Hu, K.; David, J.M. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocoll. 2015, 44, 101–108. [Google Scholar]
- Liu, Q.; Cuiping, H.; Yumeng, T.; Tongyu, L. Fabrication of curcumin-loaded zein nanoparticles stabilized by sodium caseinate/sodium alginate: Curcumin solubility, thermal properties, rheology, and stability. Process Biochem. 2020, 94, 30–38. [Google Scholar]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar]
- Xie, Z.; Zhenhai, Z.; Huixia, L. Rapamycin loaded TPGS-Lecithins-Zein nanoparticles based on core-shell structure for oral drug administration. Int. J. Pharm. 2019, 568, 118529–118539. [Google Scholar]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; McClements, D.J.; Hu, K. Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Li, H.; Dongfeng, W.; Chengzhen, L.; Junxiang, Z.; Minghao, F.; Xun, S.; Teng, W.; Ying, X.; Yanping, C. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2019, 87, 342–351. [Google Scholar]
- da Rosa, C.G.; William, G.S.; Matheus, V.; de Oliveira, B.M.; de Melo, A.P.Z.; Almeida, A.; Nunes, M.R.; Bertoldi, F.C.; Baretto, P.L.M. Development of poly (ethylene oxide) bioactive nanocomposite films functionalized with zein nanoparticles. Colloids Surf. Physicochem. Eng. Aspects 2020, 586, 124268. [Google Scholar] [CrossRef]
- Thewissen, B.G.; Inge Celus, K.B.; Jan, A.D. Foaming properties of wheat gliadin. J. Agric. Food Chem. 2011, 59, 1370–1375. [Google Scholar] [CrossRef]
- Thewissen, B.G.; Inge Celus, K.B.; Jan, A.D. Foaming properties of tryptic gliadin hydrolysate peptide fractions. Food Chem. 2011, 128, 606–612. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Iris, J.J.; Jan, A.D. Understanding the air-water interfacial behavior of suspensions of wheat gliadin nanoparticles. Food Hydrocoll. 2020, 102, 105638–105646. [Google Scholar] [CrossRef]
- Peng, D.; Weiping, J.; Jing, L.; Wenfei, X.; Yaqiong, P.; Yuntao, W.; Yan, L.; Bin, L. Adsorption and distribution of edible gliadin nanoparticles at the air/water interface. J. Agric. Food Chem. 2017, 65, 2454–2460. [Google Scholar]
- Joye, I.; Veronique, A.N.; McClements, J.D. Gliadin-based nanoparticles: Fabrication and stability of food-grade colloidal delivery systems. Food Hydrocoll. 2015, 44, 86–93. [Google Scholar]
- Wang, Y.; Wenjia, Y.; Rui, L.; Xin, J.; Yongqiang, C. Impact of deamidation on gliadin-based nanoparticle formation and curcumin encapsulation. J. Food Eng. 2019, 260, 30–39. [Google Scholar]
- Wu, W.; Xiangzhen, K.; Caimeng, Z.; Yufei, H.; Yeming, C. Improving the stability of wheat gliadin nanoparticles–Effect of gum arabic addition. Food Hydrocoll. 2018, 80, 78–87. [Google Scholar]
- Ma, L.; Liqiang, Z.; David, J.M.; Wei, L. One-step preparation of high internal phase emulsions using natural edible Pickering stabilizers: Gliadin nanoparticles/gum Arabic. Food Hydrocoll. 2020, 100, 105381–105392. [Google Scholar]
- Zhu, Y.; Qing, X.; Chen, A.; McClements, D.J.; Zou, L.; Wei, L. Pickering-stabilized emulsion gels fabricated from wheat protein nanoparticles: Effect of pH, NaCl and oil content. J. Disp. Sci. Technol. 2018, 39, 826–835. [Google Scholar]
- Chen, X.; Yan, C.; Liqiang, Z.; Xingcai, Z.; Yuqing, D.; Tang, J.; McClements, J.; Liu, W. Plant-based nanoparticles prepared from proteins and phospholipids consisting of a core–multilayer-shell structure: Fabrication, stability, and foamability. J. Agric. Food Chem. 2019, 67, 6574–6584. [Google Scholar]
- Yang, S.; Lei, D.; Cuixia, S.; Yanxiang, G. Characterization of curcumin loaded gliadin-lecithin composite nanoparticles fabricated by antisolvent precipitation in different blending sequences. Food Hydrocoll. 2018, 85, 185–194. [Google Scholar]
- Duclairoir, C.; Orecchioni, A.M.; Depraetere, P.; Nakache, E. α-Tocopherol encapsulation and in vitro release from wheat gliadin nanoparticles. J. Microencapsul. 2002, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiangzhen, K.; Caimeng, Z.; Yufei, H.; Yeming, C.; Xingfei, L. Fabrication and characterization of resveratrol-loaded gliadin nanoparticles stabilized by gum Arabic and chitosan hydrochloride. LWT 2020, 129, 109532–109542. [Google Scholar]
- Ezpeleta, I.; Juan, M.I.; Serge, S.; Christiane, C.; Jacques, G.; Yves, P.; Anne-Marie, O. Gliadin nanoparticles for the controlled release of all-trans-retinoic acid. Int. J. Pharm. 1996, 131, 191–200. [Google Scholar] [CrossRef]
- Duclairoir, C.; Orecchioni, A.-M.; Depraetere, P.; Osterstock, F.; Nakache, E. Evaluation of gliadins nanoparticles as drug delivery systems: A study of three different drugs. Int. J. Pharm. 2003, 253, 133–144. [Google Scholar] [CrossRef]
- Gulfam, M.; Ji-eun, K.; Jong, M.L.; Boram, K.; Bong, H.C.; Bong, G.C. Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir 2012, 28, 8216–8223. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Zhen, S.; Helan, X.; Yang, Y. Development of wheat glutenin nanoparticles and their biodistribution in mice. J. Biomed. Mater. Res. Part A 2015, 103, 1653–1658. [Google Scholar] [CrossRef]
- Li, F.; Chao, Q.; Man, L.; Liu, X.; Yanping, S.; Qingjie, S. Preparation and characterization of redox-sensitive glutenin nanoparticles. Int. J. Biol. Macromol. 2019, 137, 327–336. [Google Scholar]
- Teng, Z.; Yangchao, L.; Qin, W. Nanoparticles synthesized from soy protein: Preparation, characterization, and application for nutraceutical encapsulation. J. Agric. Food Chem. 2012, 60, 2712–2720. [Google Scholar]
- Wang, S.; Yuqing, L.; Xiao-kun, Q.; Junhong, L. Fabrication of soy protein isolate/cellulose nanocrystal composite nanoparticles for curcumin delivery. Int. J. Biol. Macromol. 2020, 165, 1468–1474. [Google Scholar]
- Chen, F.; Bian-Shen, L.; Chuan-He, T. Nanocomplexation of soy protein isolate with curcumin: Influence of ultrasonic treatment. Food Res. Int. 2015, 75, 157–165. [Google Scholar]
- Peng, S.; Lei, Z.; Qizhen, C.; Liqiang, Z.; Chengmei, L.; Wei, L.; David, J.M. Utilization of biopolymers to stabilize curcumin nanoparticles prepared by the pH-shift method: Caseinate, whey protein, soy protein and gum Arabic. Food Hydrocoll. 2020, 107, 105963–105974. [Google Scholar]
- Teng, Z.; Yangchao, L.; Thomas, W.; Boce, Z.; Qin, W. Development and application of nanoparticles synthesized with folic acid conjugated soy protein. J. Agric. Food Chem. 2013, 61, 2556–2564. [Google Scholar]
- Renu, K.; Kadiyala, G.; Gurunathan, J. Formulation and characterisation of antibody-conjugated soy protein nanoparticles—implications for neutralisation of snake venom with improved efficiency. Appl. Biochem. Biotechnol. 2014, 174, 2557–2570. [Google Scholar]
- Yao, W.; Qian, Z.; Xu, C.; Xin, W.; Jun, W.; Rupei, T. Folic acid-conjugated soybean protein-based nanoparticles mediate efficient antitumor ability in vitro. J. Biomater. Appl. 2017, 31, 832–843. [Google Scholar]
- Xiang, H.; Dongxiao, S.; Chun, C.; Wei, W.; Keming, D. Modification of soy protein isolate by glutaminase for nanocomplexation with curcumin. Food Chem. 2018, 268, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Feibai, Z.; Yuanhong, Z.; Dan, Y.; Qiangzhong, Z.; Mouming, Z. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocoll. 2020, 105, 105844–105853. [Google Scholar]
- Cheng, X.; Xin, W.; Zhipeng, C.; Weijing, Y.; Jun, W.; Rupei, T. Folic acid-modified soy protein nanoparticles for enhanced targeting and inhibitory. Mater. Sci. Eng. C 2017, 71, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Tina, I.L.; Wallace, Y.; Luisa, W.C.; Fang, Z. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar]
- Zhang, J.; Liang, L.; Tian, Z.; Chen, L.; Subirade, M. Preparation and in vitro evaluation of calcium-induced soy protein isolate nanoparticles and their formation mechanism study. Food Chem. 2012, 133, 390–399. [Google Scholar] [CrossRef]
- Zhang, J.; Field, C.J.; Vine, D.; Chen, L. Intestinal uptake and transport of vitamin B 12-loaded soy protein nanoparticles. Pharm. Res. 2015, 32, 1288–1303. [Google Scholar] [CrossRef]
- Chen, F.; Shi-Yi, O.; Chuan-He, T. Core–shell soy protein–soy polysaccharide complex (nano) particles as carriers for improved stability and sustained release of curcumin. J. Agric. Food Chem. 2016, 64, 5053–5059. [Google Scholar]
- Liu, L.; Peng-Zhan, L.; Xiu-Ting, L.; Ning, Z.; Chuan-He, T. Novel soy β-conglycinin core–shell nanoparticles as outstanding ecofriendly nanocarriers for curcumin. J. Agric. Food Chem. 2019, 22, 6292–6301. [Google Scholar]
- Liu, L.; Peng-Zhan, L.; Xiu-Ting, L.; Ning, Z.; Chuan-He, T. Novel soy β-conglycinin nanoparticles by ethanol-assisted disassembly and reassembly: Outstanding nanocarriers for hydrophobic nutraceuticals. Food Hydrocoll. 2019, 91, 246–255. [Google Scholar]
- Qian, X.; Lei, G.; Kangjun, Y.; Cheng, L.; Xu, Z.; Weibo, C.; Rongshi, C.; Xiqun, J. Targeting and microenvironment-improving of phenylboronic acid-decorated soy protein nanoparticles with different sizes to tumor. Theranostics 2019, 9, 7417–7425. [Google Scholar]
- Gao, Z.; Le-Ping, Z.; Xiao-Quan, Y.; Xiu-Ting, H.; Jin-Mei, W.; Jian, G.; Jun-Ru, Q.; Li-Juan, W.; Shou-Wei, Y. Soy lipophilic protein nanoparticles as a novel delivery vehicle for conjugated linoleic acid. Food Funct. 2014, 5, 1286–1293. [Google Scholar]
- Liu, F.; Chuan-He, T. Soy protein nanoparticle aggregates as pickering stabilizers for oil-in-water emulsions. J. Agric. Food Chem. 2013, 37, 8888–8898. [Google Scholar]
- Liu, F.; Shi-Yi, O.; Chuan-He, T. Ca2+- induced soy protein nanoparticles as pickering stabilizers: Fabrication and characterization. Food Hydrocoll. 2017, 65, 175–186. [Google Scholar]
- Zhu, X.; Jie, Z.; Fu, L.; Cao-Ying, Q.; Wei-Feng, L.; Chuan-He, T. Freeze-thaw stability of Pickering emulsions stabilized by soy protein nanoparticles. Influence of ionic strength before or after emulsification. Food Hydrocoll. 2018, 74, 37–45. [Google Scholar]
- Ju, M.; Gang, Z.; Guo, H.; Xinchun, S.; Yan, Z.; Lianzhou, J.; Xiaonan, S. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocoll. 2020, 99, 105329–105333. [Google Scholar]
- Li, Q.; Qixin, H.; Mengyue, X.; Junguang, L.; Xiao, L.; Zhili, W.; Xiaoquan, Y. Food-Grade Emulsions and Emulsion Gels Prepared by Soy Protein–Pectin Complex Nanoparticles and Glycyrrhizic Acid Nanofibrils. J. Agric. Food Chem. 2020, 68, 1051–1063. [Google Scholar]
- Sengupta, S.; Dipak, K.B.; Riddhi, G.; Jayati, B. Emulsions stabilized by soy protein nanoparticles as potential functional non-dairy yogurts. J. Sci. Food. Agric. 2019, 99, 5808–5818. [Google Scholar] [CrossRef] [PubMed]
- Jong, L. Characterization of soy protein nanoparticles prepared by high shear microfluidization. J. Dis. Sci. Tech. 2013, 34, 469–475. [Google Scholar]
- Gao, Z.; Jin-Mei, W.; Na-Na, W.; Zhi-li, W.; Jian, G.; Xiao-Quan, Y.; Shou-wei, Y. Formation of complex interface and stability of oil-in-water (O/W) emulsion prepared by soy lipophilic protein nanoparticles. J. Agric. Food Chem. 2013, 61, 7838–7847. [Google Scholar]
- Zhang, Y.; Feibai, Z.; Mouming, Z.; Lianzhu, L.; Zhengxiang, N.; Baoguo, S. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. 2018, 74, 62–71. [Google Scholar] [CrossRef]
- Weng, Q.; Xixi, C.; Fang, Z.; Shaoyun, W. Fabrication of self-assembled Radix Pseudostellariae protein nanoparticles and the entrapment of curcumin. Food Chem. 2019, 274, 796–802. [Google Scholar]
- Wang, R.; Zhigang, T.; Lingyun, C. Nano-encapsulations liberated from barley protein microparticles for oral delivery of bioactive compounds. Int. J. Pharm. 2011, 406, 153–162. [Google Scholar]
- Yang, J.; Ying, Z.; Lingyun, C. Elaboration and characterization of barley protein nanoparticles as an oral delivery system for lipophilic bioactive compounds. Food Funct. 2014, 5, 92–101. [Google Scholar]
- Fan, Y.; Xianxie, Z.; Jiang, Y.; Yuzhu, Z. Fabrication of pea protein nanoparticles with calcium-induced cross-linking for the stabilization and delivery of antioxidative resveratrol. Int. J. Biol. Macrmol. 2020, 152, 189–198. [Google Scholar]
- Asadi, M.; Maryam, S.; Mehdi, H.; Zahra, E.; Ali, A.; Atiyeh, G. Electrospray production of curcumin-walnut protein nanoparticles. Food Biophys. 2021, 16, 15–26. [Google Scholar] [CrossRef]
- Sneharani, A.H. Curcumin–sunflower protein nanoparticles—A potential antiinflammatory agent. J. Food Biochem. 2019, 43, e12909–e12919. [Google Scholar] [CrossRef]
- Shi, A.; Xue, C.; Li, L.; Hui, H.; Hongzhi, L.; Qiang, W.; Dominic, A. Synthesis and characterization of calcium-induced peanut protein isolate nanoparticles. RSC Adv. 2017, 84, 53247–53254. [Google Scholar]
- Ning, F.; Zhenzhen, G.; Liang, Q.; Xiaoqi, W.; Liping, L.; Hua, X.; Qingrong, H. Double-induced se-enriched peanut protein nanoparticles preparation, characterization and stabilized food-grade pickering emulsions. Food Hydrocoll. 2020, 99, 105308–105317. [Google Scholar]
- Peng, H.; Zhaodi, G.; Hua, X.; Mei, L.; Ningxiang, Y.; Tao, W.; Ronghui, W.; Yanbin, L. Self-assembly of protein nanoparticles from rice bran waste and their use as delivery system for curcumin. ACS Sustain. Chem. Eng. 2017, 5, 6605–6614. [Google Scholar]
- Ke, L.; Guan-zhen, G.; Yong, S.; Jian-wu, Z.; Ping-fan, R. Encapsulation of aconitine in self-assembled licorice protein nanoparticles reduces the toxicity in vivo. Nanoscale Res. Lett. 2015, 10, 449. [Google Scholar]
- Mirshahi, T.; Irache, J.M.C.; Nicolas, C.; Mirshahi, M.; Faure, J.P.; Gueguen, J.; Hecquet, C.; Orecchioni., A.M. Adaptive immune responses of legumin nanoparticles. J. Drug Targ. B 2002, 10, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhongzheng, C.; Yuanyuan, Z.; Xiaorong, L.; Bin, L. Novel food-grade Pickering emulsions stabilized by tea water-insoluble protein nanoparticles from tea residues. Food Hydrocoll. 2019, 96, 322–330. [Google Scholar]





















| Zein-Polysaccharide Nanoparticles | Bioactive Compounds | CDD * | Activity | References |
|---|---|---|---|---|
| Zein-pectin | Omega-3 polyunsaturated fatty | Heart disease | Antihypertensive | [23] |
| Zein-pectin | Curcumin | Different diseases | Antioxidant, anti-inflammatory, antimicrobial. Improved thermal and UV resistance, ionic strength | [24] |
| Zein-pectin | Resveratrol | Cancer | Antioxidant and anticancer | [25] |
| Zein-NaCas-pectin ** | Curcumin | Different diseases | Antioxidant | [26] |
| Zein-NaCas-pectin | Eugenol | Possible application nCDD | Antioxidant, antimicrobial | [27] |
| Zein-NaCas-pectin and Zein-NaCas-carboxymethyl cellulose zein-NaCas-gum arabic | Curcumin | Different diseases | Antioxidant | [28] |
| Zein-pectin-sodium alginate | Curcumin | Different diseases | Antioxidant | [29] |
| Zein-carboxymethyl chitosan | Vitamin D3 | Chronic diseases | Prevention | [30] |
| Zein-carboxymethyl chitosan | Indole-3-carbinol and 3,30-diindolylmethane | Cancer | Anticancer | [31] |
| Zein-chitosan | Vitamin E (α-tocopherol) | Different diseases | Antioxidant | [32] |
| Zein-chitosan | Epigallocatechin gallate | Possible application in CDD | Antioxidant | [33] |
| Zein-carboxymethyl chitosan | Polyphenols and β-carotene | Cancer and Heart disease | Antioxidant | [34] |
| Zein-quaternized chitosan | Curcumin | Possible application in CDD | Antioxidant | [35] |
| Zein-Maillard conjugates *** and Zein-NaCas-dextran | Resveratrol | Health | Nutraceutical | [36] |
| Zein-shellac | Curcumin | Possible application in CDD | Antioxidant, anti-inflammatory and anticancer | [37] |
| Zein–sodium carboxymethyl cellulose | Paclitaxel | Cancer | Anticancer | [38,39] |
| Zein-chondroitin sulphate | Sorafenib | Cancer | Anticancer | [40] |
| Applications of Nanoparticles/Payload Used | Strategies to Improve Stability | Stability Results | References |
|---|---|---|---|
| 5-fluorouracil | Formulation stored at 4 °C | Coating increased EE, nanoparticlesaggregated at low concentration of emulsifiers. | [41] |
| Thymol | Coated with caseinate and chitosan | Coated particles were stable at pH 3 to 8. | [42] |
| Mint oil | Coated with gum arabic | Uncoated particles released oil faster | [20] |
| Thymol and carvacrol | Formulation stored at 4 °C | Nanoparticles precipitated after 2 months at 20 °C but not at 4 °C | [43] |
| Resveratrol | Coated with sodium caseinate | Coating improved stability for up to 28 days | [36] |
| Lutein | Coated with lecithin and Pluronic | Coating improved stability for up to 30 days | [44] |
| Hollow zein | Thermal treatment in thermostatic water bath | Treatment at 75 °C for 15 min resulted in lower diameter and PDI | [45] |
| Hollow zein | Coated with carrageenan | Coating maintained constant diameter for 30 days at pH between 5.25 and 6.75. | [46] |
| Resveratrol | Coated with pectin | Stability was dependent on pectin concentration | [47] |
| Resveratrol | Coated with chitosan | Coating improved gastrointestinal stability and mucoadhesive properties | [48] |
| Anarcardic acid | Dimetilglyoxim | 381.6 nm particles were stable with negative ζ potential and antimicrobial activity, pH, salt and thermal stability, improved bioaccessibility and antioxidant activity. | [49] |
| Curcumin-Natamycin | 70% alginate and 30% fish gelatinCarboxymethyl chitosan | Remained stable and were resistant to neutral pH (6.0–8.0), ion strength (0–100 mM) and long-term storage (30 days). | [50] |
| Epithelial cells—293 | Chondroitin sulfate as a stabilizer. | -Stable nanoparticles between pH 3 and 8 | [51] |
| Sample | Particle Size, nm | Poly Dispersity Index (PDI) | Zeta Potential, mV | Encapsulation Efficiency, % | Loading Capacity, % | ||||
|---|---|---|---|---|---|---|---|---|---|
| before | after | before | after | before | after | before | after | ||
| Zein-curcumin | 183 ± 4 | 2031 ± 177 | 0.272 ± 0.003 | 0.369 ± 0.091 | −17.7 ± 0.2 | −2.2 ± 0.7 | 67 ± 1.3 | 4.6 ± 0.9 | 4.6 ± 0.94 |
| 358 ± 11 | 876 ± 12 | 0.234 ± 0.002 | 0.331 ± 0.021 | −25.6 ± 0.4 | −19.1 ± 2.0 | 79.4 ± 2.1 | 4.9 ± 1.0 | 4.9 ± 1.0 | |
| 230 ± 24 | 209 ± 5 | 0.286 ± 0.009 | 0.213 ± 0.029 | −35.3 ± 0.6 | −39.3 ± 1.0 | 76.1 ± 0.9 | 4.8 ± 1.2 | 4.8 ± 1.2 | |
| 239 ± 1 | 226 ± 11 | 0.314 ± 0.004 | 0.196 ± 0.025 | −41.7 ± 0.7 | −41.5 ± 0.5 | 72.8 ± 1.5 | 4.7 ± 0.9 | 4.7 ± 0.9 | |
| Nanoparticle | Average Size, nm | Zeta Potential, mV | Drug Loading, % |
|---|---|---|---|
| 7% gliadin | 218.66 ± 5.1 | 18.46 ± 8.3 | 72.02 ± 5.6 |
| 7% gliadin, 4% gelatin | 398.56 ± 4.2 | 19.00 ± 3.6 | 64.23 ± 8.9 |
| 7% gliadin, 8% gelatin | 450.10 ± 9.7 | 14.20 ± 3.7 | 52.77 ± 12.6 |
| Curcumin/Protein Ratio | Encapsulation Efficiency, % | Loading Efficiency, % | Particle Size, nm | Zeta Potential, mV |
|---|---|---|---|---|
| Pure proteins | - | - | 201.5 ± 9.2 | −36.8 ± 1.0 |
| 1% curcumin | 97.2 ± 2.0 | 1.1 ± 0.1 | 220.1 ± 17.8 | −36.0 ± 2.1 |
| 2% curcumin | 81.2 ± 1.2 | 1.7 ± 0.1 | 252.6 ± 13.4 | −35.2 ± 0.8 |
| 3% curcumin | 52.8 ± 3.0 | 2.7 ± 0.2 | 286.7 ± 10.1 | −34.5 ± 1.4 |
| Parameter | Curcumin | Untreated Soy Protein | Enzyme Treated Soy Protein | ||
|---|---|---|---|---|---|
| 1% | 8% | 1% | 8% | ||
| Encapsulation Efficiency, % | - | 96.7 ± 0.27 | 97.6 ± 0.21 | 97.3 ± 0.30 | 98.4 ± 0.07 |
| Loading amount, µg/mg | - | 36.2 ± 0.93 | 25.7 ± 0.56 | 101 ± 2.41 | 34.9 ± 0.06 |
| Diameter, nm | No Yes | 86.4 ± 1.05 105 ± 0.76 | 402 ± 0.15 547 ± 0.38 | 113 ± 1.27 142 ± 1.05 | 523 ± 0.78 692 ± 2.10 |
| Zeta potential, mV | No Yes | −31.1 ± 0.85 −38.4 ± 0.27 | −32.3 ± 1.27 −37.7 ± 1.81 | −8.5 ± 0.82 −30.9 ± 1.61 | −11.1 ± 0.16 −30.6 ± 1.13 |
| Hydrophobicity | No Yes | 3472 ± 6.51 2779 ± 0.99 | 3101 ± 1.56 2926 ± 2.55 | 3827 ± 4.17 2926 ± 2.55 | 4176 ± 2.47 2534 ± 3.61 |
| CaCl2 (mM) | pH | Diameter, nm | PDI | Zeta Potential, mV | pH | Size, nm | Zeta Potential, mV | ||
|---|---|---|---|---|---|---|---|---|---|
| 2.5 mM | 5 mM | 2.5 mM | 5 mM | ||||||
| 2.5 | 7 | 60 ± 3 | 0.28 ± 0.00 | −11.7 ± 0.3 | 2 | 33.9 ± 2.4 | 82.8 ± 1.3 | 16.9 ± 0.9 | 17.3 ± 2.3 |
| - | 8 | 28 ± 1 | 0.27 ± 0.00 | −14.3 ± 0.7 | 3 | 45.9 ± 5.6 | 99.4 ± 1.4 | 18.7 ± 0.6 | 19.3 ± 1.5 |
| 5 | 8 | 101 ± 2 | 0.29 ± 0.00 | −8.8 ± 0.6 | 7 | 100.8 ± 14.7 | 146.0 ± 13.3 | −10.3 ± 0.4 | −9.5 ± 2.1 |
| - | 9 | 71 ± 3 | 0.23 ± 0.03 | −11.0 ± 0.3 | 8 | 32.7 ± 7.2 | 119.0 ± 35.1 | −15.8 ± 1.1 | −10.8 ± 2.3 |
| 10 | 9 | 179 ± 3 | 0.27 ± 0.00 | −7.5 ± 0.6 | 9 | 29 ± 10.2 | 76.0 ± 0.5 | −19.1 ± 0.2 | −11.4 ± 1.7 |
| Parameter | pH | CaCl2 Concentration | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 mM | 1 mM | 2.5 mM | 3.5 mM | 5 mM | 7.5 mM | 10 mM | ||
| Z-ave (nm) | 7 | 134.1 ± 0.5 b | 125.5 ± 0.9 a | 173.1 ± 1.8 c | 429.2 ± 17.8 d | 5470.7 ± 1032.1 e,f | 4474.0 ± 400.0 e | 5870.7 ± 355.6 f |
| 8 | 142.4 ± 0.4 b | 138.3 ± 1.2 a | 157.9 ± 1.9 c | 191.2 ± 4.2 d | 455.5 ± 8.6 e | 6414.0 ± 809.8 f | 5873.3 ± 771.5 f | |
| 9 | 143.0 ± 0.3 a | 146.2 ± 1.5 b | 176.6 ± 2.5 c | 202.3 ± 6.6 d | 194.8 ± 1.5 d | 247.2 ± 1.3 e | 799.2 ± 36.4 f | |
| Turbidity | 7 | 0.432 ± 0.015 a | 0.494 ± 0.009 b | 1.215 ± 0.021 c | 3.072 ± 0.045 d | 3.298 ± 0.035 e | 3.307 ± 0.043 e | 3.303 ± 0.055 e |
| 8 | 0.416 ± 0.021 a | 0.478 ± 0.014 b | 0.802 ± 0.017 c | 1.268 ± 0.023 d | 2.790 ± 0.037 e | 3.232 ± 0.030 f | 3.260 ± 0.042 f | |
| 9 | 0.408 ± 0.012 a | 0.428 ± 0.011 a | 0.615 ± 0.010 b | 0.921 ± 0.010 d | 0.892 ± 0.015 c | 1.861 ± 0.022 e | 3.065 ± 0.037 f | |
| PDI | 7 | 0.245 ± 0.004 a | 0.265 ± 0.002 b | 0.257 ± 0.004 b | 0.573 ± 0.094 c | 0.421 ± 0.183 c | 0.422 ± 0.066 c | 0.458 ± 0.070 c |
| 8 | 0.259 ± 0.011 a | 0.250 ± 0.010 a | 0.363 ± 0.015 b | 0.372 ± 0.037 b | 0.410 ± 0.092 b | 0.633 ± 0.075 c | 0.597 ± 0.036 c | |
| 9 | 0.245 ± 0.008 a | 0.260 ± 0.005 a | 0.309 ± 0.034 b | 0.474 ± 0.058 c | 0.423 ± 0.007 c | 0.442 ± 0.008 c | 0.997 ± 0.006 d | |
| Zeta-potential (mV) | 7 | −21.90 ± 2.15 f | −16.70 ± 1.78 e | −13.90 ± 0.75 d | −11.40 ± 0.80 c | −8.39 ± 0.41 b | −4.98 ± 0.54 a | −4.64 ± 0.45 a |
| 8 | −26.27 ± 1.40 e | −23.90 ± 1.15 d | −21.10 ± 1.97 d | −16.67 ± 1.36 c | −14.53 ± 1.36 c | −10.54 ± 0.55 b | −8.52 ± 0.83 a | |
| 9 | −29.17 ± 2.20 e | −25.77 ± 1.31 e | −19.63 ± 1.68 d | −15.87 ± 1.00 c | −13.17 ± 1.31 c | −10.69 ± 0.97 b | −7.72 ± 0.85 a | |
| Sample | Unloaded NPs | Curcumin Loaded NPs |
|---|---|---|
| Concentration, % w/w | 3.5 | 3.5 |
| Curcumin:WPI ratio, % w/w | - | 0.111111111 |
| Apparent viscosity mPaS | 12.25 ± 3.35 | 6.35 ± 0.32 |
| Electrical conductivity (μS/cm) | 177.12 ± 7.14 | 133.5 ± 6.13 |
| Zeta potential (mV) | −14.6 ± 2.01 | −17.7 ± 0.94 |
| Voltage (kV) | 20 | 20 |
| Tip to collector distance (cm) | 17.5 | 17.5 |
| Flow rate (mL/h) | 0.09 | 0.09 |
| Nozzle diameter | 18 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, N.; Rapisarda, M. Properties and Applications of Nanoparticles from Plant Proteins. Materials 2021, 14, 3607. https://doi.org/10.3390/ma14133607
Reddy N, Rapisarda M. Properties and Applications of Nanoparticles from Plant Proteins. Materials. 2021; 14(13):3607. https://doi.org/10.3390/ma14133607
Chicago/Turabian StyleReddy, Narendra, and Marco Rapisarda. 2021. "Properties and Applications of Nanoparticles from Plant Proteins" Materials 14, no. 13: 3607. https://doi.org/10.3390/ma14133607