Spongostan™ Leads to Increased Regeneration of a Rat Calvarial Critical Size Defect Compared to NanoBone® and Actifuse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bone Substitute Materials
2.2. Animals
2.3. Animal Surgery
2.4. Postoperative Care
2.5. Euthanasia and Sample Extraction
2.6. Micro-CT
2.7. Statistics
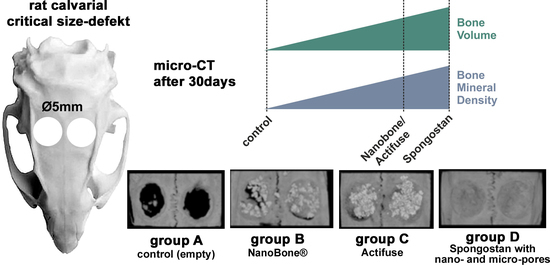
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauceri, R.; Murgia, D.; Cicero, O.; Paterno, L.; Fiorillo, L.; De Caro, V.; Campisi, G. Leucocyte-and platelet-rich fibrin block: Its use for the treatment of a large cyst with implant-based rehabilitation. Medicina 2021, 57, 180. [Google Scholar] [CrossRef]
- Stramandinoli-Zanicotti, R.T.; Sassi, L.M.; Rebelatto, C.L.; Boldrine-Leite, L.M.; Brofman, P.R.; Carvalho, A.L. The effect of bone marrow-derived stem cells associated with platelet-rich plasma on the osseointegration of immediately placed implants. J. Clin. Exp. Dent. 2021, 13, e8–e13. [Google Scholar] [CrossRef]
- Amaroli, A.; Colombo, E.; Zekiy, A.; Aicardi, S.; Benedicenti, S.; De Angelis, N. Interaction between laser light and osteoblasts: photobiomodulation as a trend in the management of socket bone preservation—A review. Biology 2020, 9, 409. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Craniomaxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Osorio, C.C.; Escobar, L.M.; Gonzalez, M.C.; Gamboa, L.F.; Chambrone, L. Evaluation of density, volume, height and rate of bone resorption of substitutes of autologous bone grafts for the repair of alveolar clefts in humans: A systematic review. Heliyon 2020, 6, e04646. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Riccitiello, F.; Rengo, S.; Marrelli, B.; Valletta, R.; Spagnuolo, G. Management of endodontic and periodontal lesions: The role of regenerative dentistry and biomaterials. Dent. J. 2020, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco-Ortega, E.; Valente, N.A.; Iezzi, G.; Petrini, M.; Derchi, G.; Barone, A. Maxillary sinus augmentation with three different biomaterials: Histological, histomorphometric, clinical, and patient-reported outcomes from a randomized controlled trial. Clin. Implant Dent. Relat. Res. 2021, 23, 86–95. [Google Scholar] [CrossRef]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658. [Google Scholar] [CrossRef]
- Stumbras, A.; Januzis, G.; Gervickas, A.; Kubilius, R.; Juodzbalys, G. Randomized and controlled clinical trial of bone healing after alveolar ridge preservation using xenografts and allografts versus plasma rich in growth factors. J. Oral Implantol. 2020, 46, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.F.; Gottschalk, M.; Fokin, N.; Buker, B.; Kaltschmidt, B.P.; Dreyer, A.; Vordemvenne, T.; Kaltschmidt, C.; Hutten, A.; Kaltschmidt, B. Natural and synthetic nanopores directing osteogenic differentiation of human stem cells. Nanomedicine 2019, 17, 319–328. [Google Scholar] [CrossRef]
- Petersen, A.; Princ, A.; Korus, G.; Ellinghaus, A.; Leemhuis, H.; Herrera, A.; Klaumunzer, A.; Schreivogel, S.; Woloszyk, A.; Schmidt-Bleek, K.; et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat. Commun. 2018, 9, 4430. [Google Scholar] [CrossRef] [Green Version]
- Vordemvenne, T.; Wahnert, D.; Koettnitz, J.; Merten, M.; Fokin, N.; Becker, A.; Buker, B.; Vogel, A.; Kronenberg, D.; Stange, R.; et al. Bone regeneration: A novel osteoinductive function of Spongostan by the interplay between its nano- and microtopography. Cells 2020, 9, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.S.; Park, C.H.; Hong, S.L.; Kim, M.J.; Kim, J.Y.; Kim, Y.W.; Koo, S.K.; Roh, H.J. Comparative analysis of Cutanplast and Spongostan nasal packing after endoscopic sinus surgery: A prospective, randomized, multicenter study. Eur. Arch. Otorhinolaryngol. 2015, 272, 1699–1705. [Google Scholar] [CrossRef]
- Cegielski, M.; Izykowska, I.; Podhorska-Okolow, M.; Zabel, M.; Dziegiel, P. Development of foreign body giant cells in response to implantation of Spongostan as a scaffold for cartilage tissue engineering. In Vivo 2008, 22, 203–206. [Google Scholar] [PubMed]
- De Concilio, B.; Vedovo, F.; Mir, M.C.; Silvestri, T.; Casarin, A.; Celia, A. Gelatin sponge (Spongostan(R)) and N-butyl-2-cyanoacrylate: Utility on percutaneous treatment of persistent urinary leakage after partial nephrectomy. Case report and review of the literature. Arch. Ital. Urol. Androl. 2020, 92. [Google Scholar] [CrossRef]
- Simurda, T.; Dobrotova, M.; Skornova, I.; Sokol, J.; Kubisz, P.; Stasko, J. Successful use of a highly purified plasma von Willebrand factor concentrate containing little FVIII for the long-term prophylaxis of severe (Type 3) von Willebrand’s disease. Semin. Thromb. Hemost. 2017, 43, 639–641. [Google Scholar]
- Arias-Gallo, J.; Chamorro-Pons, M.; Avendano, C.; Gimenez-Gallego, G. Influence of acidic fibroblast growth factor on bone regeneration in experimental cranial defects using spongostan and Bio-Oss as protein carriers. J. Craniofac. Surg. 2013, 24, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Chang, Y.H.; Li, K.C.; Lu, C.H.; Sung, L.Y.; Yeh, C.L.; Lin, K.J.; Huang, S.F.; Yen, T.C.; Hu, Y.C. The use of ASCs engineered to express BMP2 or TGF-beta3 within scaffold constructs to promote calvarial bone repair. Biomaterials 2013, 34, 9401–9412. [Google Scholar] [CrossRef] [PubMed]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petrie, A.; Cimoes, R.; Donos, N. A systematic review on the critical size defect model. Clin. Oral Implant. Res. 2014, 25, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Mardas, N.; Dereka, X.; Stavropoulos, A.; Patel, M.; Donos, N. The role of strontium ranelate and guided bone regeneration in osteoporotic and healthy conditions. J. Periodontal Res. 2021, 56, 330–338. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendviliene, I.; Simoliunas, E.; Alksne, M.; Dibart, S.; Jasiuniene, E.; Cicenas, V.; Jacobs, R.; Bukelskiene, V.; Rutkunas, V. Effect of extracellular matrix and dental pulp stem cells on bone regeneration with 3D printed PLA/HA composite scaffolds. Eur. Cells Mater. 2021, 41, 204–215. [Google Scholar] [CrossRef]
- Nakamura, T.; Shirakata, Y.; Shinohara, Y.; Miron, R.J.; Hasegawa-Nakamura, K.; Fujioka-Kobayashi, M.; Noguchi, K. Comparison of the effects of recombinant human bone morphogenetic protein-2 and -9 on bone formation in rat calvarial critical-size defects. Clin. Oral Investig. 2017, 21, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Gerber, T.; Holzhüter, G.; Götz, W.; Bienengräber, V.; Henkel, K.-O.; Rumpel, E. Nanostructuring of biomaterials—A pathway to bone grafting substitute. Eur. J. Trauma 2006, 32, 132–140. [Google Scholar] [CrossRef]
- Götz, W.; Gerber, T.; Michel, B.; Lossdörfer, S.; Henkel, K.-O.; Heinemann, F. Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone®) osteogenesis: A study on biopsies from human jaws. Clin. Oral Implant. Res. 2008, 19, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Korzinskas, T.; Chia, P.; Maawi, S.A.; Eichler, K.; Sader, R.A.; Ghanaati, S. Do clinical and radiological assessments contribute to the understanding of biomaterials? Results from a prospective randomized sinus augmentation split-mouth trial. J. Oral Implantol. 2018, 44, 62–69. [Google Scholar] [CrossRef]
- Abshagen, K.; Schrodi, I.; Gerber, T.; Vollmar, B. In vivo analysis of biocompatibility and vascularization of the synthetic bone grafting substitute NanoBone®. J. Biomed. Mater. Res. Part A 2009, 91A, 557–566. [Google Scholar] [CrossRef]
- Licina, P.; Coughlan, M.; Johnston, E.; Pearcy, M. Comparison of silicate-substituted calcium phosphate (actifuse) with recombinant human bone morphogenetic protein-2 (infuse) in posterolateral instrumented lumbar fusion. Glob. Spine J. 2015, 5, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valliant, E.M.; Jones, J.R. Softening bioactive glass for bone regeneration: Sol–gel hybrid materials. Soft Matter 2011, 7, 5083–5095. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Jenis, L.G.; Kovach, M.E.; Marini, J.; Turner, A.S. Efficacy of silicated calcium phosphate graft in posterolateral lumbar fusion in sheep. Spine J. 2007, 7, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 2003, 24, 4609–4620. [Google Scholar] [CrossRef]
- Shanbhag, S.; Pandis, N.; Mustafa, K.; Nyengaard, J.R.; Stavropoulos, A. Bone tissue engineering in oral peri-implant defects in preclinical in vivo research: A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2018, 12, e336–e349. [Google Scholar] [CrossRef]
- Kruijt Spanjer, E.C.; Bittermann, G.K.P.; van Hooijdonk, I.E.M.; Rosenberg, A.; Gawlitta, D. Taking the endochondral route to craniomaxillofacial bone regeneration: A logical approach? J. Craniomaxillofac. Surg. 2017, 45, 1099–1106. [Google Scholar] [CrossRef]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Hennocq, Q.; Bennedjai, A.; Simon, F.; Testelin, S.; Devauchelle, B.; Tulasne, J.F.; Dakpe, S.; Khonsari, R.H. Maxillofacial surgery in wartime Middle-East: Paul Tessier’s missions to Iran. J. Craniomaxillofac. Surg. 2019, 47, 1449–1455. [Google Scholar] [CrossRef]
- Kadam, D. Salvage secondary reconstruction of the mandible with vascularized fibula flap. Craniomaxillofac. Trauma Reconstr. 2019, 12, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.C. Gene/environment causes of cleft lip and/or palate. Clin. Genet. 2002, 61, 248–256. [Google Scholar] [CrossRef]
- Tan, B.; Tang, Q.; Zhong, Y.; Wei, Y.; He, L.; Wu, Y.; Wu, J.; Liao, J. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int. J. Oral Sci. 2021, 13, 9. [Google Scholar] [CrossRef]
- Peters, F.; Kniha, K.; Mohlhenrich, S.C.; Bock, A.; Holzle, F.; Modabber, A. Evaluation of a novel osteosynthesis plate system for mandibular defects. Br. J. Oral Maxillofac. Surg. 2020, 58, e109–e114. [Google Scholar] [CrossRef] [PubMed]
- Prisby, R.D. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J. Endocrinol. 2017, 235, R77–R100. [Google Scholar] [CrossRef] [Green Version]
- Santolini, E.; West, R.; Giannoudis, P.V. Risk factors for long bone fracture non-union: A stratification approach based on the level of the existing scientific evidence. Injury 2015, 46 (Suppl. 8), S8–S19. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Silva, M.J. Skeletal blood flow in bone repair and maintenance. Bone Res. 2013, 1, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Friis, T.; Glatt, V.; Crawford, R.; Xiao, Y. Structural properties of fracture haematoma: Current status and future clinical implications. J. Tissue Eng. Regen. Med. 2017, 11, 2864–2875. [Google Scholar] [CrossRef] [PubMed]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannoudis, P.V.; Gudipati, S.; Harwood, P.; Kanakaris, N.K. Long bone non-unions treated with the diamond concept: A case series of 64 patients. Injury 2015, 46 (Suppl. 8), S48–S54. [Google Scholar] [CrossRef]
- Miska, M.; Findeisen, S.; Tanner, M.; Biglari, B.; Studier-Fischer, S.; Grutzner, P.A.; Schmidmaier, G.; Moghaddam, A. Treatment of nonunions in fractures of the humeral shaft according to the Diamond Concept. Bone Joint J. 2016, 98, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, A.; Thaler, B.; Bruckner, T.; Tanner, M.; Schmidmaier, G. Treatment of atrophic femoral non-unions according to the diamond concept: Results of one- and two-step surgical procedure. J. Orthop. 2017, 14, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for bone regeneration: From graft to tissue engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Li, P.; Liu, J.; Peng, S. Optimization of TCP/HAP ratio for better properties of calcium phosphate scaffold via selective laser sintering. Mater. Charact. 2013, 77, 23–31. [Google Scholar] [CrossRef]
- Li, C.; Jiang, C.; Deng, Y.; Li, T.; Li, N.; Peng, M.; Wang, J. RhBMP-2 loaded 3D-printed mesoporous silica/calcium phosphate cement porous scaffolds with enhanced vascularization and osteogenesis properties. Sci. Rep. 2017, 7, 41331. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc silicate/nano-hydroxyapatite/collagen scaffolds promote angiogenesis and bone regeneration via the p38 MAPK pathway in activated monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Macdougall, L.; Culver, H.; Lin, C.-C.; Bowman, C.; Anseth, K. 1.3.2F—Degradable and resorbable polymers. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 167–190. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Dahabreh, Z.; Panteli, M.; Pountos, I.; Howard, M.; Campbell, P.; Giannoudis, P.V. Ability of bone graft substitutes to support the osteoprogenitor cells: An in-vitro study. World J. Stem Cells 2014, 6, 497–504. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C. Nanomaterial-based bone regeneration. Nanoscale 2017, 9, 4862–4874. [Google Scholar] [CrossRef]
- Teo, B.K.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.S.; Yap, C.H.; Ramagopal, Y.; Romer, L.H.; Yim, E.K.F. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Rybak, Z.; Szymonowicz, M.; Wiglusz, R.J. Selected nanomaterials’ application enhanced with the use of stem cells in acceleration of alveolar bone regeneration during augmentation process. Nanomaterials 2020, 10, 1216. [Google Scholar] [CrossRef]
- Cornell, C.N. Osteoconductive materials and their role as substitutes for autogenous bone grafts. Orthop. Clin. N. Am. 1999, 30, 591–598. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Weinstein, A.M. The status of porous materials to obtain direct skeletal attachment by tissue ingrowth. Acta Orthop. Belg. 1974, 40, 755–765. [Google Scholar]
- Ghayor, C.; Weber, F.E. Osteoconductive microarchitecture of bone substitutes for bone regeneration revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef]
- Weber, F.E. Reconsidering osteoconduction in the era of additive manufacturing. Tissue Eng. Part B Rev. 2019, 25, 375–386. [Google Scholar] [CrossRef]
- Li, S.; De Wijn, J.R.; Li, J.; Layrolle, P.; De Groot, K. Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Eng. 2003, 9, 535–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seebach, C.; Schultheiss, J.; Wilhelm, K.; Frank, J.; Henrich, D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury 2010, 41, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Augustin, G.; Davila, S.; Mihoci, K.; Udiljak, T.; Vedrina, D.S.; Antabak, A. Thermal osteonecrosis and bone drilling parameters revisited. Arch. Orthop. Trauma Surg. 2008, 128, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Tu, Y.K.; Tsai, Y.J.; Tsai, Y.S.; Yen, C.Y.; Yang, S.C.; Hsiao, C.K. Assessment of thermal necrosis risk regions for different bone qualities as a function of drilling parameters. Comput. Methods Programs Biomed. 2018, 162, 253–261. [Google Scholar] [CrossRef] [PubMed]





| New Bone Volume | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| Mean | 2.97 | 6.69 | 6.03 | 7.98 |
| Median | 2.56 | 6.79 | 6.26 | 8.12 |
| Standard deviation | 1.31 | 1.70 | 1.03 | 1.68 |
| Minimum | 1.70 | 2.44 | 3.74 | 4.94 |
| Maximum | 6.17 | 8.75 | 7.37 | 1.83 |
| Group A | Group B | Group C | Group D | |||||
|---|---|---|---|---|---|---|---|---|
| BMD | New | Intact | New | Intact | New | Intact | New | Intact |
| Mean | 0.77 | 0.67 | 0.73 | 0.67 | 0.69 | 0.64 | 0.80 | 0.61 |
| Median | 0.78 | 0.68 | 0.73 | 0.67 | 0.69 | 0.62 | 0.80 | 0.61 |
| Standard deviation | 0.04 | 0.04 | 0.03 | 0.05 | 0.07 | 0.07 | 0.02 | 0.06 |
| Minimum | 0.71 | 0.60 | 0.68 | 0.60 | 0.61 | 0.57 | 0.78 | 0.51 |
| Maximum | 0.82 | 0.74 | 0.77 | 0.75 | 0.79 | 0.76 | 0.83 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wähnert, D.; Koettnitz, J.; Merten, M.; Kronenberg, D.; Stange, R.; Greiner, J.F.W.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. Spongostan™ Leads to Increased Regeneration of a Rat Calvarial Critical Size Defect Compared to NanoBone® and Actifuse. Materials 2021, 14, 1961. https://doi.org/10.3390/ma14081961
Wähnert D, Koettnitz J, Merten M, Kronenberg D, Stange R, Greiner JFW, Kaltschmidt C, Vordemvenne T, Kaltschmidt B. Spongostan™ Leads to Increased Regeneration of a Rat Calvarial Critical Size Defect Compared to NanoBone® and Actifuse. Materials. 2021; 14(8):1961. https://doi.org/10.3390/ma14081961
Chicago/Turabian StyleWähnert, Dirk, Julian Koettnitz, Madlen Merten, Daniel Kronenberg, Richard Stange, Johannes F. W. Greiner, Christian Kaltschmidt, Thomas Vordemvenne, and Barbara Kaltschmidt. 2021. "Spongostan™ Leads to Increased Regeneration of a Rat Calvarial Critical Size Defect Compared to NanoBone® and Actifuse" Materials 14, no. 8: 1961. https://doi.org/10.3390/ma14081961