Heterostructured CoOx–TiO2 Mesoporous/Photonic Crystal Bilayer Films for Enhanced Visible-Light Harvesting and Photocatalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bilayer Film Deposition and Surface Modification
2.2. Material Characterization
2.3. Photocatalytic Performance
3. Results and Discussion
3.1. Morphological, Structural, and Optical Properties
3.2. Optical Properties
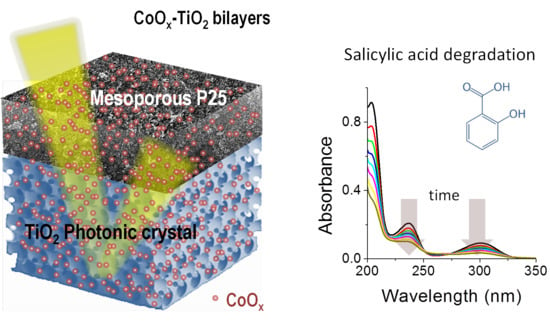
3.3. Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phillips, K.R.; England, G.T.; Sunny, S.; Shirman, E.; Shirman, T.; Vogel, N.; Aizenberg, J. A colloidoscope of colloid-based porous materials and their uses. Chem. Soc. Rev. 2016, 45, 281–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B: Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Yu, J.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. TiO2 inverse opal photonic crystals: Synthesis, modification, and applications—A review. J. Alloys Compd. 2018, 769, 740–757. [Google Scholar] [CrossRef]
- Chen, J.I.L.; von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Amplified photochemistry with slow photons. Adv. Mater. 2006, 18, 1915–1919. [Google Scholar] [CrossRef]
- Curti, M.; Schneider, J.; Bahnemann, D.W.; Mendive, C.B. Inverse opal photonic crystals as a strategy to improve photocatalysis: Underexplored questions. J. Phys. Chem. Lett. 2015, 6, 903–3910. [Google Scholar] [CrossRef]
- Pang, F.; Jiang, Y.; Zhang, Y.; He, M.; Ge, J. Synergetic enhancement of photocatalytic activity with a photonic crystal film as a catalyst support. J. Mater. Chem. A 2015, 3, 21439–21443. [Google Scholar] [CrossRef]
- Li, P.; Chen, S.L.; Wang, A.J.; Wang, Y. Probing photon localization effect between titania and photonic crystals on enhanced photocatalytic activity of titania film. Chem. Eng. J. 2016, 284, 305–314. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, F.; Pang, F.; Ge, J. Substantial Enhancement toward the photocatalytic activity of CdS quantum dots by photonic crystal-supporting films. ACS Appl. Mater. Interfaces 2018, 10, 42241–42248. [Google Scholar] [CrossRef]
- Jelle, A.A.; Ghuman, K.K.; O’Brien, P.G.; Hmadeh, M.; Sandhel, A.; Perovic, D.D.; Singh, C.V.; Mims, C.A.; Ozin, G.A. Highly Efficient ambient temperature CO2 photomethanation catalyzed by nanostructured RuO2 on silicon photonic crystal support. Adv. Energy Mater. 2018, 8, 1702277. [Google Scholar] [CrossRef]
- Xie, C.; Fan, T.; Wang, A.; Chen, S.L. Enhanced visible-light photocatalytic activity of a TiO2 membrane-assisted with N-doped carbon quantum dots and SiO2 opal photonic crystal. Ind. Eng. Chem. Res. 2019, 58, 120–127. [Google Scholar] [CrossRef]
- Nishimura, S.; Abrams, N.; Lewis, B.A.; Halaoui, L.I.; Mallouk, T.E.; Benkstein, K.D.; van de Lagemaat, J.; Frank, A.J. Standing wave enhancement of red absorbance and photocurrent in dye-sensitized titanium dioxide photoelectrodes coupled to photonic crystals. J. Am. Chem. Soc. 2003, 125, 6306–6310. [Google Scholar] [CrossRef] [PubMed]
- Halaoui, L.; Abrams, N.; Mallouk, T. Increasing the conversion efficiency of dye-sensitized tio2 photoelectrochemical cells by coupling to photonic crystals. J. Phys. Chem. B 2005, 109, 6334–6342. [Google Scholar] [CrossRef] [PubMed]
- Guldin, S.; Hüttner, S.; Kolle, M.; Welland, M.E.; Müller-Buschbaum, P.; Friend, R.H.; Tétreault, N.; Steiner, U. Dye-sensitized solar cell based on a three-dimensional photonic crystal. Nano Lett. 2010, 10, 2303–2309. [Google Scholar] [CrossRef] [Green Version]
- Tada, H.; Jin, Q.; Iwaszuk, A.; Nolan, M. Molecular-scale transition metal oxide nanocluster surface-modified titanium dioxide as solar-activated environmental catalysts. J. Phys. Chem. C 2014, 118, 12077–12086. [Google Scholar] [CrossRef]
- Maeda, K.; Ishimaki, K.; Okazaki, M.; Kanazawa, T.; Lu, D.; Nozawa, S.; Kato, H.; Kakihana, M. Cobalt oxide nanoclusters on rutile titania as bifunctional units for water oxidation catalysis and visible light absorption: Understanding the structure—Activity relationship. ACS Appl. Mater. Interfaces 2017, 9, 6114–6122. [Google Scholar] [CrossRef] [PubMed]
- Šuligoj, A.; Arčon, I.; Mazaj, M.; Dražić, G.; Arčon, D.; Cool, P.; Štangar, U.L.; Tušar, N.N. Surface modified titanium dioxide using transition metals: Nickel as a winning transition metal for solar light photocatalysis. J. Mater. Chem. A 2018, 6, 9882–9892. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ke, J.; Li, Y.; Liu, B.; Wang, L.; Xiao, H.; Wang, S. Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2018, 236, 396–403. [Google Scholar] [CrossRef]
- Schubert, J.S.; Popovic, J.; Haselmann, G.M.; Nandan, S.P.; Wang, J.; Giesriegl, A.; Cherevan, A.S.; Eder, D. Immobilization of Co, Mn, Ni and Fe oxide co-catalysts on TiO2 for photocatalytic water splitting reactions. J. Mater. Chem. A 2019, 7, 18568–18579. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, M.; Wang, Y.; Yokoi, T.; Maeda, K. Visible-light driven water oxidation using anatase titania modified with first row transition-metal-oxide nanoclusters. J. Phys. Chem. C 2019, 123, 10429–10434. [Google Scholar] [CrossRef]
- Tanaka, H.; Uchiyama, T.; Kawakami, N.; Okazaki, M.; Uchimoto, Y.; Maeda, K. Water oxidation through interfacial electron transfer by visible light using cobalt-modified rutile titania thin-film photoanode. ACS Appl. Mater. Interfaces 2020, 12, 9219–9225. [Google Scholar] [CrossRef]
- Tada, H.; Jin, Q.; Nishijima, H.; Yamamoto, H.; Fujishima, M.; Okuoka, S.-i.; Hattori, T.; Sumida, Y.; Kobayashi, H. Titanium(IV) dioxide surface-modified with iron oxide as a visible light photocatalyst. Angew. Chem. Int. Ed. 2011, 50, 3501–3505. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.; Iwaszuk, A.; Tada, H. Molecular metal oxide cluster-surface modified titanium(IV) dioxide photocatalysts. Aust. J. Chem. 2012, 65, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.; Yamamoto, H.; Yamamoto, K.; Fujishima, M.; Tada, H. Simultaneous induction of high level thermal and visible-light catalytic activities to titanium(IV) oxide by surface modification with cobalt(III) oxide clusters. Phys. Chem. Chem. Phys. 2013, 15, 20313–20319. [Google Scholar] [CrossRef]
- Hatton, B.; Mishchenko, L.; Davis, S.; Sandhage, K.H.; Aizenberg, J. Assembly of large-area, highly ordered, crack-free inverse opal films. Proc. Natl. Acad. Sci. USA 2010, 107, 10354–10359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamantopoulou, A.; Sakellis, E.; Romanos, G.E.; Gardelis, S.; Ioannidis, N.; Boukos, N.; Falaras, P.; Likodimos, V. Titania photonic crystal photocatalysts functionalized by graphene oxide nanocolloids. Appl. Catal. B Environ. 2019, 240, 277–290. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Nazeeruddin, M.K.; Liska, P.; Pechy, P.; Grätzel, M. Fabrication of screen-printing pastes from TiO2 powders for dye-sensitised solar cells. Prog. Photovolt: Res. Appl. 2007, 15, 603–612. [Google Scholar] [CrossRef]
- Tunesi, S.; Anderson, M. Influence of chemisorption on the photodecomposition of salicylic acid and related compounds using suspended TiO2 ceramic membranes. J. Phys. Chem. 1991, 95, 3399–3405. [Google Scholar] [CrossRef]
- Diamantopoulou, A.; Sakellis, E.; Gardelis, S.; Tsoutsou, D.; Glenis, S.; Boukos, N.; Dimoulas, A.; Likodimos, V. Advanced photocatalysts based on reduced nanographene oxide–TiO2 photonic crystal films. Materials 2019, 12, 2518. [Google Scholar] [CrossRef] [Green Version]
- Ishimaki, K.; Uchiyama, T.; Okazaki, M.; Lu, D.; Uchimoto, Y.; Maeda, K. Influence of TiO2 support on activity of Co3O4/TiO2 photocatalysts for visible-light water oxidation. Bull. Chem. Soc. Jpn. 2018, 91, 486–491. [Google Scholar] [CrossRef]
- Balaji, S.; Djaoued, Y.; Robichaud, J. Phonon confinement studies in nanocrystalline anatase-TiO2 thin films by micro Raman spectroscopy. J. Raman Spectrosc. 2006, 37, 1416–1422. [Google Scholar] [CrossRef]
- Likodimos, V.; Stergiopoulos, T.; Falaras, P.; Kunze, J.; Schmuki, P. Phase composition, size, orientation, and antenna effects of self-assembled anodized titania nanotube arrays: A polarized micro-Raman investigation. J. Phys. Chem. C 2008, 112, 12687–12696. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO-Co3O4 crossover using Raman spectroscopy in magnetic octahedron shaped nanocrystals. J. Raman Spectrosc. 2017, 48, 837–841. [Google Scholar] [CrossRef]
- Qiao, L.; Xiao, H.Y.; Meyer, H.M.; Sun, J.N.; Rouleau, C.M.; Puretzky, A.A.; Geohegan, D.B.; Ivanov, I.N.; Yoon, M.; Weber, W.J. Nature of the band gap and origin of the electro-/photo-activity of Co3O4. J. Mater. Chem. C 2013, 1, 4628–4633. [Google Scholar] [CrossRef]
- Li, Y.; Qui, W.; Qin, F.; Fang, H.; Hadjiev, V.G.; Litvinov, D.; Bao, J. Identification of cobalt oxides with Raman scattering and Fourier transform infrared spectroscopy. J. Phys. Chem. C 2016, 120, 4511–4516. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, H.; Huang, X.; Mo, Y.; Chen, L. Impacts of electrolyte solvent soakage on structure and electrochemical performance of LiCoO2 for lithium-ion batteries. Electrochem. Solid-State Lett. 2004, 7, A353–A357. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. XPS studies of solvated metal atom dispersed catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- Toumazatou, A.; Antoniadou, M.; Sakellis, E.; Tsoutsou, D.; Gardelis, S.; Romanos, G.; Ioannidis, N.; Boukos, N.; Dimoulas, A.; Falaras, P.; et al. Boosting visible light harvesting and charge separation in surface modified TiO2 photonic crystal catalysts by CoOx nanoclusters. Mater. Adv. 2020. [Google Scholar] [CrossRef]
- Mayer, J.T.; Diebold, U.; Madey, T.E.; Garfunkel, E. Titanium and reduced titania overlayers on titanium dioxide(110). J. Electron Spectrosc. Relat. Phenom. 1995, 73, 1–11. [Google Scholar] [CrossRef]
- Singh, V.; Major, D.T. Electronic structure and bonding in Co-based single and mixed valence oxides: A quantum chemical perspective. Inorg. Chem. 2016, 55, 3307–3315. [Google Scholar] [CrossRef]
- Toumazatou, A.; Arfanis, M.K.; Pantazopoulos, P.-A.; Kontos, A.G.; Falaras, P.; Stefanou, N.; Likodimos, V. Slow-photon enhancement of dye sensitized TiO2 photocatalysis. Mater. Lett. 2017, 197, 123–126. [Google Scholar] [CrossRef]
- Regazzoni, A.E.; Mandelbaum, P.; Matsuyoshi, M.; Schiller, S.; Bilmes, S.A.; Blesa, M.A. Adsorption and photooxidation of salicylic acid on titanium dioxide: A surface complexation description. Langmuir 1998, 14, 868–874. [Google Scholar] [CrossRef]
- Arfanis, M.K.; Adamou, P.; Moutakas, N.G.; Theodoros, M.T.; Kontos, A.G.; Falaras, P. Photocatalytic degradation of salicylic acid and caffeine emerging contaminants using titania nanotubes. Chem. Eng. J. 2017, 310, 525–536. [Google Scholar] [CrossRef]
- Guinea, E.; Arias, C.; Cabot, P.L.; Garrido, J.A.; Rodríguez, R.M.; Centellas, F.; Brillas, E. Mineralization of salicylic acid in acidic aqueous medium by electrochemical advanced oxidation processes using platinum and boron-doped diamond as anode and cathodically generated hydrogen peroxide. Water Res. 2008, 42, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Cherdhirankorn, T.; Retsch, M.; Jonas, U.; Butt, H.-J.; Koynov, K. Tracer diffusion in silica inverse opals. Langmuir 2010, 26, 10141–10146. [Google Scholar] [CrossRef] [PubMed]
- Raccis, R.; Nikoubashman, A.; Retsch, M.; Jonas, U.; Koynov, K.; Butt, H.-J.; Likos, C.N.; Fytas, G. Confined diffusion in periodic porous nanostructures. ACS Nano 2011, 5, 4607–4616. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.N.; Barako, M.T.; Tice, J.; Won, Y. Microscale liquid transport in polycrystalline inverse opals across grain boundaries. Sci. Rep. 2017, 7, 10465. [Google Scholar] [CrossRef] [Green Version]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukopoulos, S.; Toumazatou, A.; Sakellis, E.; Xenogiannopoulou, E.; Boukos, N.; Dimoulas, A.; Likodimos, V. Heterostructured CoOx–TiO2 Mesoporous/Photonic Crystal Bilayer Films for Enhanced Visible-Light Harvesting and Photocatalysis. Materials 2020, 13, 4305. https://doi.org/10.3390/ma13194305
Loukopoulos S, Toumazatou A, Sakellis E, Xenogiannopoulou E, Boukos N, Dimoulas A, Likodimos V. Heterostructured CoOx–TiO2 Mesoporous/Photonic Crystal Bilayer Films for Enhanced Visible-Light Harvesting and Photocatalysis. Materials. 2020; 13(19):4305. https://doi.org/10.3390/ma13194305
Chicago/Turabian StyleLoukopoulos, Stelios, Alexia Toumazatou, Elias Sakellis, Evangelia Xenogiannopoulou, Nikos Boukos, Athanasios Dimoulas, and Vlassis Likodimos. 2020. "Heterostructured CoOx–TiO2 Mesoporous/Photonic Crystal Bilayer Films for Enhanced Visible-Light Harvesting and Photocatalysis" Materials 13, no. 19: 4305. https://doi.org/10.3390/ma13194305