Synergistic Influences of Stearic Acid Coating and Recycled PET Microfibers on the Enhanced Properties of Composite Materials
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Recycled PET Fibers Pretreatment
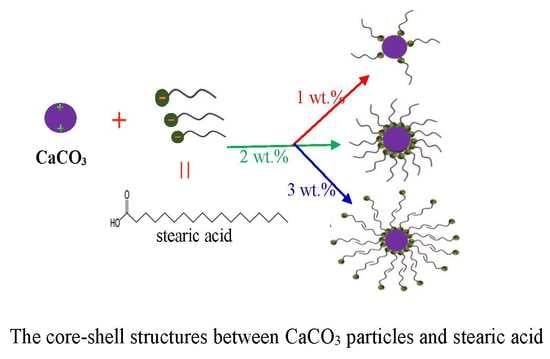
2.3. Modification of CaCO3 Surface by Stearic Acid
2.4. Preparation of Epoxy/CaCO3/recycled PET Fibers Mat
2.5. Material Characterizations
3. Results and Discussion
3.1. Recycled PET Fibers Treatment
3.2. CaCO3 Modification by Stearic Acid
3.3. Morphology of Artificial Marble Materials
3.4. Mechanical Properties of Artificial Marble Materials
3.4.1. Influent of Stearic Acid and Recycled PET Fibers
3.4.2. Influent of Stearic Acid Contents
3.5. Thermal Decomposition of Artificial Marble Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rostami, R.; Zarrebini, M.; Mandegari, M.; Mostofinejad, D.; Mahdi Abtahi, S.M. A review on performance of polyester fibers in alkaline and cementitious composites environments. Constr. Build. Mater. 2020, 241, 117998. [Google Scholar] [CrossRef]
- Merli, R.; Preziosi, M.; Acampora, A.; Lucchetti, C.M.; Petrucci, E. Recycled fibers in reinforced concrete: A systematic literature review. J. Clean. Prod. 2020, 248, 119207. [Google Scholar] [CrossRef]
- Bai, Y.L.; Yan, Z.W.; Ozbakkaloglu, T.; Han, Q.; Dai, J.G.; Zhu, D.J. Quasi-static and dynamic tensile properties of large-rupture-strain (LRS) polyethylene terephthalate fiber bundle. Constr. Build. Mater. 2020, 232, 117241. [Google Scholar] [CrossRef]
- Manuel, J.C.C.; Gaxiola, A.; Alvarado-Beltrán, C.G.; Orozco-Carmona, V.M.; Pellegrini-Cervantes, M.J.; Rodríguez-Rodríguez, M.; Castro-Beltrán, A. A new application of recycled-PET/PAN composite nanofibers to cement–based materials. J. Clean. Prod. 2020, 252, 119827. [Google Scholar]
- Bertelsen, I.M.G.; Ottosen, L.M.; Fischer, G. Influence of fibre characteristics on plastic shrinkage cracking in cement-based materials: A review. Constr. Build. Mater. 2020, 230, 116769. [Google Scholar] [CrossRef]
- Shariatmadari, N.; Karimpour-Fard, M.; Hasanzadehshooiili, H.; Hoseinzadeh, S.; Karimzadeh, Z. Effects of drainage condition on the stress-strain behavior and pore pressure buildup of sand-PET mixtures. Constr. Build. Mater. 2020, 233, 117295. [Google Scholar] [CrossRef]
- Yesilata, B.; Isıker, Y.; Turgut, P. Thermal insulation enhancement in concretes byadding waste PET and rubber pieces. Constr. Build. Mater. 2009, 23, 1878–1882. [Google Scholar] [CrossRef]
- Panyakapo, P.; Panyakapo, M. Reuse of thermosetting plastic waste for lightweight concrete. Waste Manag. 2008, 28, 1581–1588. [Google Scholar] [CrossRef]
- Ochi, T.; Okubo, S.; Fukui, K. Development of recycled PET fiber and its application as concrete- reinforcing fiber. Cem. Concr. Compos. 2007, 29, 448–455. [Google Scholar] [CrossRef]
- Wang, Y.J.; Backer, S.; Li, V.C. An experimental study of synthetic fiber reinforced cementitious composites. J. Mater. Sci. 1987, 22, 4260–4281. [Google Scholar] [CrossRef]
- Kim, J.H.G.; Ja, P.C.; Lee, S.W.; Won, J.P. Effects of the geometry of recycled PET fiber reinforcement on shrinkage cracking of cement-based composites. Compos. Part B Eng. 2008, 39, 442–450. [Google Scholar] [CrossRef]
- Won, J.P.; Jang, C.-I.; Lee, S.-W.; Lee, S.J.; Kim, H.-Y. Long-term performance of recycled PET fibre-reinforced cement composites. Constr. Build. Mater. 2010, 24, 660–665. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, S. Building Decorative Materials; Wiley & Son: Hoboken, NJ, USA, 2011. [Google Scholar]
- Li, L.; Zou, H.; Shao, L.; Wang, G.; Chen, J. Study on mechanical property of epoxy composites filled with nano-sized calcium carbonate perticles. J. Mater. Sci. 2005, 40, 1297–1299. [Google Scholar] [CrossRef]
- He, H.; Li, K.; Wang, J.; Sun, G.; Li, Y.; Wang, J. Study on thermal and mechnical properties of nano-calcium carbonate/epoxy composites. Mater. Des. 2011, 32, 4521–4527. [Google Scholar] [CrossRef]
- He, H.; Zhang, Z.; Wang, J.; Li, K. Compressive properties of nano-calcium carbonate/epoxy and its fibre composites. Compos. Part B Eng. 2013, 45, 919–924. [Google Scholar] [CrossRef]
- Yogurtcuoglu, E.; Ucurum, M. Surface modification of calcite by wet-stirredball milling and its properties. Powder Technol. 2011, 214, 47–53. [Google Scholar] [CrossRef]
- Mihajlovic, S.R.; Vucinic, D.R.; Sekulic, T.Z.; Mili’cevic, S.Z.; Kolonja, B.M. Mechanism of stearic acid adsorption to calcite. Powder Technol. 2013, 245, 208–216. [Google Scholar] [CrossRef]
- Lam, T.D.; Hoang, T.V.; Quang, D.T.; Kim, J.S. Effect of nanosized andsurface-modified precipitated calcium carbonate on properties ofCaCO3/polypropylene nanocomposites. Mater. Sci. Eng. A 2009, 501, 87–93. [Google Scholar] [CrossRef]
- Osman, M.A.; Atallah, A.; Suter, U.W. Influence of excessive filler coating on thetensile properties of LDPE–calcium carbonate composites. Polymer 2004, 45, 1177–1183. [Google Scholar] [CrossRef]
- Rosmaninho, M.G.; Jarrdim, E.; Moura, F.C.C.; Ferreira, G.L.; Thim, V.; Yoshida, M.I.; Araujo, M.H.; Lago, R.M. Surface hydrolysis of postconsumer Polyethyelen Terephthalate to procude adsorbents for cationic acontaminants. Appl. Polym. Sci. 2006, 102, 5284–5291. [Google Scholar] [CrossRef]
- Khoshnevis, H.; Zadhoush, A. The Influence of Epoxy resin On The Morphological and Rheological Properties of PET/PA66 Blend. Rheol. Acta 2012, 51, 467–480. [Google Scholar] [CrossRef]
- Qu, C.; Yang, H.; Liang, D.; Cao, W.; Fu, Q. Morphology and Properties of PET/PA-6/SiO2 Ternary Composites. Appl. Polym. Sci. 2006, 104, 2288–2296. [Google Scholar] [CrossRef]
- Wilinski, D.; Lukowski, P.; Rokicki, G. Application of fibers from recycled PET bottles for concrete reinforcement. J. Build. Chem. 2016, 1, 1–9. [Google Scholar]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds; Wiley–Interscience: New York, NY, USA, 1970. [Google Scholar]
- Cai, G.B.; Chen, S.F.; Liu, L.; Jiang, J.; Tao, H.B.; Xu, A.W.; Yu, S.H. 1,3-Diamino-2-hydroxypropane-N,N,N’,N’-etraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. CrystEngComm 2009, 12, 234–241. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Zhong, X.W.; Liu, Z.Q.; Chen, S.; Li, N. Preparation and enhancement of thermal conductivity of heat transfer oil-based MoS2 nanofluids. J. Nanomater. 2013, 13, 1–6. [Google Scholar]
- Tran, H.-V.; Tran, D.L.; Vu, D.H.; Hoang, T. Facile Surface Modification of nanoprecipitated calcium carbonate by adsorption of sodium stearate in aqueous solution. Collids Surf. A Physicochem. Eng. Asp. 2010, 95–103. [Google Scholar] [CrossRef]
- Fekete, E.; Pukánszky, B.; Tóth, A.; Bertóti, I. Surface modification and characterization of particulate mineral fillers. J. Colloid Interface Sci. 1990, 135, 200–208. [Google Scholar] [CrossRef]
- Cao, Z.; Daly, M.; Clémence, L.; Geever, L.M.; Major, I.; Higginbotham, C.; Devine, D.M. Chemical surface modification of calcium carbonate particles withstearic acid using different treating methods. Appl. Surf. Sci. 2016, 378, 320–329. [Google Scholar] [CrossRef]
- Zuiderduin, W.C.J.; Westzaan, C.; Huétink, J.; Gaymans, R.J. Toughening ofpolypropylene with calcium carbonate particles. Polymer 2003, 44, 261–275. [Google Scholar] [CrossRef]
- Deshmukh, G.; Pathak, S.; Peshwe, D.; Ekhe, J. Effect of uncoated calciumcarbonate and stearic acid coated calcium carbonate on mechanical, thermaland structural properties of poly(butylene terephthalate) (PBT)/calciumcarbonate composites. Bull. Mater. Sci. 2010, 33, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Alberti, M.G.; Enfedaque, A.; Gálvez, J.C. Fibre reinforced concrete with a combination of polyolefin and steel-hooked fibres. Compos. Struct. 2017, 171, 317–325. [Google Scholar] [CrossRef]
- Mazaheripour, H.; Ghanbarpour, S.; Mirmoradi, S.H.; Hosseinpour, I. The effect of polypropylene fibers on the properties of fresh and hardened lightweight selfcompacting concrete. Constr. Build. Mater. 2011, 25, 351–358. [Google Scholar] [CrossRef]
- Corinaldesi, V.; Moriconi, G. Characterization of self-compacting concretes prepared with different fibers and mineral additions. Cem. Concr. Compos. 2011, 33, 596–601. [Google Scholar] [CrossRef]
- Ghernouti, Y.; Rabehi, B.; Bouziani, T.; Ghezraoui, H.; Makhloufi, A. Fresh and hardened properties of self-compacting concrete containing plastic bag waste fibers (WFSCC). Constr. Build. Mater. 2015, 1, 89–100. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Tran, D.T.; Nguyen, T.T.; Grillet, A.C.; Kim, N.H.; Lee, J.H. Enhanced Mechanical and Thermal Properties of Recycled ABS/Nitrile Rubber/Nanofil N15 Nanocomposites. Compos. Part B Eng. 2016, 93, 280–288. [Google Scholar]
- Sahmaran, M.; Yaman, I.O. Hybrid fiber reinforced self-compacting concrete with a high-volume coarse fly ash. Constr. Build. Mater. 2007, 21, 150–156. [Google Scholar] [CrossRef]
- Farnam, Y.; Mohammadi, S.; Shekarchi, M. Experimental and numerical investigations of low velocity impact behavior of high-performance fiber-reinforced cement based composite. Int. J. Impact Eng. 2010, 37, 220–229. [Google Scholar] [CrossRef]
- Mastali, M.; Dalvand, A.; Sattarifard, A. The impact resistance and mechanical properties of reinforced self-compacting concrete with recycled glass fibre reinforced polymers. J. Clean. Prod. 2016, 124, 312–324. [Google Scholar] [CrossRef]
- Gencel, O.; Brostow, W.; Datashvili, T.; Thedford, M. Workability and mechanical performance of steel fiber-reinforced self-compacting concrete with fly ash. Compos. Interfaces 2011, 18, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Meddah, M.S.; Bencheikh, M. Properties of concrete reinforced with different kinds of industrial waste fibre materials. Constr. Build. Mater. 2009, 23, 3196–3205. [Google Scholar] [CrossRef]
- Picazo, A.; Gálvez, J.C.; Alberti, M.G.; Enfedaque, A. Assessment of the shear behaviour of polyolefin fibre reinforced concrete and verification by means of digital image correlation. Constr. Build. Mater. 2018, 30, 565–578. [Google Scholar] [CrossRef]
- Jin, C.; Zhou, L.; Fu, L.; Zhu, J.; Li, D. Synthesis and discharge performances of NiCl2 by surface modification of carbon coating as cathode material of thermal battery. Appl. Surf. Sci. 2017, 402, 308–313. [Google Scholar] [CrossRef]










| Materials | Epoxy/TETA (9:1) (g) | CaCO3 (g) | Stearic Acid (g) | Recycled PET Fibers Treated by NaOH (g) |
|---|---|---|---|---|
| E100% | 50.0 | 0.0 | - | - |
| E60% | 30.0 | 20.0 | - | - |
| E60%S2% | 30.0 | 19.6 | 0.4 | |
| E60%S2%-PET-Na | 30.0 | 19.6 | 0.4 | 3.3 |
| Material | Begin Temperature (°C) | End Temperature (°C) | Mass Loss (%) |
|---|---|---|---|
| Epoxy | 330 | 416 | 93.2 |
| CaCO3 | 695 | - | - |
| E60% | 324 | 381 | 54.5 |
| E60%S2% | 316 | 364 | 55 |
| E60%S2%-PET-Na | 329 | 395 | 54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.M.; Vu, T.N.; Nguyen, T.M.L.; Nguyen, T.D.; Thuc, C.N.H.; Bui, Q.B.; Colin, J.; Perré, P. Synergistic Influences of Stearic Acid Coating and Recycled PET Microfibers on the Enhanced Properties of Composite Materials. Materials 2020, 13, 1461. https://doi.org/10.3390/ma13061461
Nguyen DM, Vu TN, Nguyen TML, Nguyen TD, Thuc CNH, Bui QB, Colin J, Perré P. Synergistic Influences of Stearic Acid Coating and Recycled PET Microfibers on the Enhanced Properties of Composite Materials. Materials. 2020; 13(6):1461. https://doi.org/10.3390/ma13061461
Chicago/Turabian StyleNguyen, Dang Mao, Thi Nhung Vu, Thi Mai Loan Nguyen, Trinh Duy Nguyen, Chi Nhan Ha Thuc, Quoc Bao Bui, Julien Colin, and Patrick Perré. 2020. "Synergistic Influences of Stearic Acid Coating and Recycled PET Microfibers on the Enhanced Properties of Composite Materials" Materials 13, no. 6: 1461. https://doi.org/10.3390/ma13061461