Surface-Enhanced Absorption Spectroscopy for Optical Fiber Sensing
Abstract
:1. Introduction
2. Theory
3. Materials and Methods
3.1. Fiber Preparation
3.2. Absorption Spectroscopy Measurements
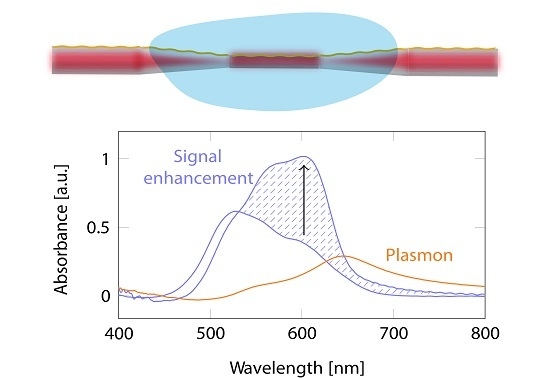
4. Results
4.1. Ideal Au Thickness
4.2. Rhodamine 6G Measurements
4.3. Crystal Violet Measurements
4.4. Comparison of the Dyes
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Vis | visible |
| NIR | near-infrared |
| MIR | mid-infrared |
| SERS | surface-enhanced Raman spectroscopy |
| PEF | plasmon-enhanced fluorescence |
| SNR | signal-to-noise ratio |
| SEIRAS | surface-enhanced infrared absorption spectroscopy |
| ATR | attenuated total reflection |
| SPR | surface plasmon resonance |
| MM | multi mode |
| LSPR | localized surface plasmon resonance |
| R6G | Rhodamine 6G |
| CV | crystal violet |
| TM | Transverse Magnetic |
| PCR | principal component regression |
| PLSR | partial least squares regression |
| ANN | artificial neural networks |
References
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; Wiley-VCH: Weinheim, Germany, 2002; Chapter 13.1; pp. 1–11, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives: A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef] [PubMed]
- Neubrech, F.; Huck, C.; Weber, K.; Pucci, A.; Giessen, H. Surface-Enhanced Infrared Spectroscopy Using Resonant Nanoantennas. Chem. Rev. 2017, 117, 5110–5145. [Google Scholar] [CrossRef] [PubMed]
- Keiser, G.; Xiong, F.; Cui, Y.; Shum, P.P. Review of diverse optical fibers used in biomedical research and clinical practice. J. Biomed. Opt. 2014, 19, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Chen, H.; Ming, T.; Zhao, L.; Wang, F.; Sun, L.D.; Wang, J.; Yan, C.H. Plasmon–molecule interactions. Nano Today 2010, 5, 494–505. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.Y.; Wang, H.; Gao, B.R.; Hao, Y.W.; Jin, Y.; Chen, Q.D.; Sun, H.B. Surface Plasmon Enhanced Fluorescence of Dye Molecules on Metal Grating Films. J. Phys. Chem. C 2011, 115, 12636–12642. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [Green Version]
- Hartstein, A.; Kirtley, J.R.; Tsang, J.C. Enhancement of the Infrared Absorption from Molecular Monolayers with Thin Metal Overlayers. Phys. Rev. Lett. 1980, 45, 201–204. [Google Scholar] [CrossRef]
- Enders, D.; Nagao, T.; Pucci, A.; Nakayama, T.; Aono, M. Surface-enhanced ATR-IR spectroscopy with interface-grown plasmonic gold-island films near the percolation threshold. Phys. Chem. Chem. Phys. 2011, 13, 4935–4941. [Google Scholar] [CrossRef]
- Brown, L.V.; Zhao, K.; King, N.; Sobhani, H.; Nordlander, P.; Halas, N.J. Surface-enhanced infrared absorption using individual cross antennas tailored to chemical moieties. J. Am. Chem. Soc. 2013, 135, 3688–3695. [Google Scholar] [CrossRef]
- Srajer, J.; Schwaighofer, A.; Ramer, G.; Rotter, S.; Guenay, B.; Kriegner, A.; Knoll, W.; Lendl, B.; Nowak, C. Double-layered nanoparticle stacks for surface enhanced infrared absorption spectroscopy. Nanoscale 2014, 6, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Bibikova, O.; Haas, J.; Lopez-Lorente, A.I.; Popov, A.; Kinnunen, M.; Ryabchikov, Y.; Kabashin, A.; Meglinski, I.; Mizaikoff, B. Surface enhanced infrared absorption spectroscopy based on gold nanostars and spherical nanoparticles. Anal. Chim. Acta 2017, 990, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubrech, F.; Pucci, A.; Cornelius, T.W.; Karim, S.; García-Etxarri, A.; Aizpurua, J. Resonant Plasmonic and Vibrational Coupling in a Tailored Nanoantenna for Infrared Detection. Phys. Rev. Lett. 2008, 101, 157403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatta, A.; Suzuki, Y.; Suëtaka, W. Infrared absorption enhancement of monolayer species on thin evaporated Ag films by use of a Kretschmann configuration: Evidence for two types of enhanced surface electric fields. Appl. Phys. A 1984, 35, 135–140. [Google Scholar] [CrossRef]
- Osawa, M. Dynamic Processes in Electrochemical Reactions Studied by Surface-Enhanced Infrared Absorption Spectroscopy (SEIRAS). Bull. Chem. Soc. Jpn. 1997, 70, 2861–2880. [Google Scholar] [CrossRef]
- Jorgenson, R.; Yee, S. A fiber-optic chemical sensor based on surface plasmon resonance. Sens. Actuators B 1993, 12, 213–220. [Google Scholar] [CrossRef]
- Johansen, K.; Arwin, H.; Lundström, I.; Liedberg, B. Imaging surface plasmon resonance sensor based on multiple wavelengths: Sensitivity considerations. Rev. Sci. Instrum. 2000, 71, 3530–3538. [Google Scholar] [CrossRef]
- Tsai, W.H.; Tsao, Y.C.; Lin, H.Y.; Sheu, B.C. Cross-point analysis for a multimode fiber sensor based on surface plasmon resonance. Opt. Lett. 2005, 30, 2209. [Google Scholar] [CrossRef]
- Golosovsky, M.; Lirtsman, V.; Yashunsky, V.; Davidov, D.; Aroeti, B. Midinfrared surface-plasmon resonance: A novel biophysical tool for studying living cells. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef] [Green Version]
- Navarrete, M.C.; Díaz-Herrera, N.; González-Cano, A.; Esteban, Ó. Surface plasmon resonance in the visible region in sensors based on tapered optical fibers. Sens. Actuators B. Chem. 2014, 190, 881–885. [Google Scholar] [CrossRef]
- Oliverio, M.; Perotto, S.; Messina, G.C.; Lovato, L.; De Angelis, F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, Z.; Payne, F. Demonstration of an optimised evanescent field optical fibre sensor. Anal. Chim. Acta 1994, 293, 49–54. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: Berlin, Germany, 2007; Chapter 1–3; pp. 5–52. [Google Scholar]
- Lirtsman, V.; Golosovsky, M.; Davidov, D. Infrared surface plasmon resonance technique for biological studies. J. Appl. Phys. 2008, 103, 014702. [Google Scholar] [CrossRef] [Green Version]
- Homola, J. Electromagnetic Theory of Surface Plasmons. In Surface Plasmon Resonance Based Sensors; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–44. [Google Scholar] [CrossRef]
- Snyder, A.W.; Love, J.D. Optical Waveguide Theory; Springer: Berlin/Heidelberg, Germany, 1983; pp. 227, 705. [Google Scholar] [CrossRef]
- Hao, E.; Schatz, G.C. Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 2004, 120, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Read, T.; Olkhov, R.V.; Shaw, A.M. Measurement of the localised plasmon penetration depth for gold nanoparticles using a non-invasive bio-stacking method. Phys. Chem. Chem. Phys. 2013, 15, 6122–6127. [Google Scholar] [CrossRef]
- Osawa, M. Surface-Enhanced Infrared Absorption. In Near-Field Optics and Surface Plasmon Polaritons; Springer: Berlin/Heidelberg, Germany, 2001; pp. 163–187. [Google Scholar] [CrossRef]
- Li, J.F.; Li, C.Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef]
- Horikoshi, S.; Matsumoto, N.; Omata, Y.; Kato, T. Growth of Au nanoparticle films and the effect of nanoparticle shape on plasmon peak wavelength. J. Appl. Phys. 2014, 115. [Google Scholar] [CrossRef]
- Xu, G.; Tazawa, M.; Jin, P.; Nakao, S. Surface plasmon resonance of sputtered Ag films: Substrate and mass thickness dependence. Appl. Phys. A 2005, 80, 1535–1540. [Google Scholar] [CrossRef]
- Terdale, S.; Tantray, A. Spectroscopic study of the dimerization of rhodamine 6G in water and different organic solvents. J. Mol. Liq. 2017, 225, 662–671. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Olmon, R.L.; Slovick, B.; Johnson, T.W.; Shelton, D.; Oh, S.H.; Boreman, G.D.; Raschke, M.B. Optical dielectric function of gold. Phys. Rev. B 2012, 86, 235147. [Google Scholar] [CrossRef] [Green Version]
- Masel, R.I. Principles of Adsorption and Reaction on Solid Surfaces; Wiley: Hoboken, NJ, USA, 1996; Chapter 4.1–4.5; pp. 235–254. [Google Scholar]
- Murai, S.; Oka, S.; Azzam, S.I.; Kildishev, A.V.; Ishii, S.; Tanaka, K. Enhanced absorption and photoluminescence from dye-containing thin polymer film on plasmonic array. Opt. Express 2019, 27, 5083–5096. [Google Scholar] [CrossRef] [PubMed]
- Wanzenböck, H.D.; Mizaikoff, B.; Weissenbacher, N.; Kellner, R. Multiple internal reflection in surface enhanced infrared absorption spectroscopy (SEIRA) and its significance for various analyte groups. J. Mol. Struct. 1997, 410–411, 535–538. [Google Scholar] [CrossRef]
- Leite, I.; Navarrete, M.C.; Díaz-Herrera, N.; González-Cano, A.; Esteban, Ó. Selectivity of SPR fiber sensors in absorptive media: An experimental evaluation. Sens. Actuators B. Chem. 2011, 160, 592–597. [Google Scholar] [CrossRef]
- Armin, A.; Soltanolkotabi, M.; Feizollah, P. On the pH and concentration response of an evanescent field absorption sensor using a coiled-shape plastic optical fiber. Sens. Actuators A 2011, 165, 181–184. [Google Scholar] [CrossRef]
- Duan, M.; Wu, J.; Xiong, Y.; Fang, S.; Chen, J. Characterization and differentiation of the adsorption behavior of crystal violet and methylene blue at the silica/water interface using near field evanescent wave. Soft Matter 2018, 14, 7516–7525. [Google Scholar] [CrossRef]
- Agelet, L.E.; Hurburgh, C.R.H., Jr. A Tutorial on Near Infrared Spectroscopy and Its Calibration. Crit. Rev. Anal. Chem. 2010, 40, 246–260. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuglerud, S.S.; Milenko, K.; Aksnes, A.; Hjelme, D.R. Surface-Enhanced Absorption Spectroscopy for Optical Fiber Sensing. Materials 2020, 13, 34. https://doi.org/10.3390/ma13010034
Fuglerud SS, Milenko K, Aksnes A, Hjelme DR. Surface-Enhanced Absorption Spectroscopy for Optical Fiber Sensing. Materials. 2020; 13(1):34. https://doi.org/10.3390/ma13010034
Chicago/Turabian StyleFuglerud, Silje S., Karolina Milenko, Astrid Aksnes, and Dag R. Hjelme. 2020. "Surface-Enhanced Absorption Spectroscopy for Optical Fiber Sensing" Materials 13, no. 1: 34. https://doi.org/10.3390/ma13010034