Spent Coffee Grounds in the Production of Lightweight Clay Ceramic Aggregates in View of Urban and Agricultural Sustainable Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. SCGs Collection, Drying, and Characterization
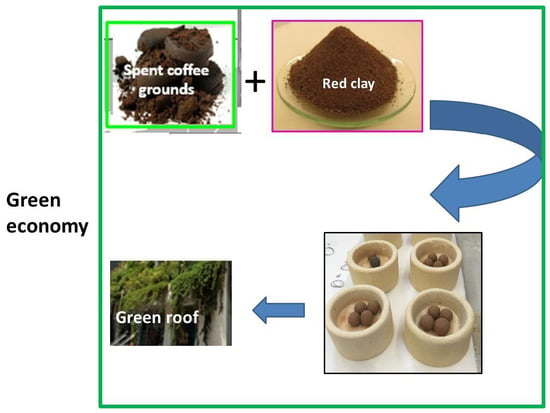
2.2. LWAs Preparation
2.3. LWAS Characterization
2.4. LWAS Release Test
3. Results and Discussion
3.1. LWAs Characterization and Ceramic Properties
3.2 Release Test of LWAs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Coffee Organization. Total Coffee Production 1990–Present. 2019. Available online: http://www.ico.org/new_historical.asp?section=Statistics (accessed on 29 October 2019).
- European Coffee Federation. European Coffee Report 2017–2018. 2019. Available online: https://www.ecf-coffee.org/publications/european-coffee-report (accessed on 29 October 2019).
- Comitato Italiano del Caffè. Esportazione Caffè 2017. 2019. Available online: http://comitcaf.it/index.php/esportazione-caffe/ (accessed on 29 October 2019).
- Allesina, G.; Pedrazzi, S.; Lovato, F.; Allegretti, F.; Tartarini, P.; Siligardi, C. Discussion of possible coffee grounds disposal chains for energy production. In Proceedings of the 23rd EUBCE (European Biomass Conference and Exhibition), Wien, Austria, 1–4 June 2015. [Google Scholar]
- Kim, J.; Kim, H.; Baek, G.; Lee, C. Anaerobic co-digestion of spent coffee grounds with different waste feedstocks for biogas production. Waste Manag. 2017, 60, 322–328. [Google Scholar] [CrossRef]
- Neves, L.; Oliveira, R.; Alves, M.M. Anaerobic co-digestion of coffee waste and sewage sludge. Waste Manag. 2006, 26, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of different rates of spent coffee grounds (SCG) on composting process, gaseous emissions and quality of end-product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Givens, D.L.; Barber, W.P. In vivo evaluation of spent coffee grounds as a ruminant feed. Agric. Wastes. 1986, 18, 69–72. [Google Scholar] [CrossRef]
- Hardgrove, S.; Livesley, S. Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Page, J.C.; Arruda, N.P.; Freitas, S.P. Crude ethanolic extract from spent coffee grounds: Volatile and functional properties. Waste Manag. 2017, 69, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.S.; Oliveira, L.S.; Ferreira, M.E. Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination. 2009, 249, 267–272. [Google Scholar] [CrossRef]
- Kua, T.; Arulrajah, A.; Horpibulsuk, S.; Du, Y.; Shen, S. Strength assessment of spent coffee grounds-geopolymer cement utilizing slag and fly ash precursors. Constr. Build. Mater. 2016, 115, 565–575. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent coffee grounds as a versatile source of green energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef] [PubMed]
- Allesina, G.; Pedrazzi, S.; Tebianian, S.; Tartarini, P. Biodiesel and electrical power production through vegetable oil extraction and byproducts gasification: Modeling of the system. Bioresour. Technol. 2014, 170, 278–285. [Google Scholar] [CrossRef]
- Pedrazzi, S.; Allesina, G.; Tartarini, P. Aige conference: A kinetic model for a stratified downdraft gasifier. Int. J. Heat Technol. 2012, 30, 41–44. [Google Scholar]
- Allesina, G.; Pedrazzi, S.; Tartarini, P. Modeling and investigation of the channeling phenomenon in downdraft stratified gasifiers. Bioresour. Technol. 2013, 146, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Allesina, G.; Pedrazzi, S.; Sgarbi, F.; Pompeo, E.; Roberti, C.; Cristiano, V.; Tartarini, P. Approaching sustainable development through energy management, the case of Fongo Tongo, Cameroon. Int. J. Energy Environ. Eng. 2015, 6, 121–127. [Google Scholar] [CrossRef]
- Allesina, G.; Pedrazzi, S.; Allegretti, F.; Tartarini, P. Spent coffee grounds as heat source for coffee roasting plant: Experimental validation and case study. Appl. Therm. Eng. 2017, 126, 730–736. [Google Scholar] [CrossRef]
- ANEFA. 2018. Available online: http://www.aridos.org/el-sector/ (accessed on 29 October 2019).
- Brown, T.J.; Idoine, N.E.; Raycraft, E.R.; Shaw, R.A.; Hobbs, S.F.; Everett, P.; Deady, E.A.; Bide, T. World Mineral Production 2012–2016; British Geological Survey: Keyworth Nottingam, UK, 2018; ISBN 978-0-85272-882-6. [Google Scholar]
- Bettzieche, H.; Schops, W.; Hohmann, H. Pore-forming in brickmaking clay by means of expanded glass granules. Ziegelindustrie. 2000, 5, 41–53. [Google Scholar]
- Laterlite. Laterlite Geothecnical Manual. 2019. Available online: https://www.laterlite.es/wp-content/uploads/2015/09/Geotechnical-Manual_2015.pdf (accessed on 29 October 2019).
- Shafique, M.; Kim, R.; Rafiq, M. Green roof benefits, opportunities and challenges—A review. Renew. Sustain. Energy Rev. 2018, 90, 757–773. [Google Scholar] [CrossRef]
- Decree, L. Decreto Legislativo n. 75, 29 Aprile 2010: Riordino e revisione della disciplina in materia di fertilizzanti, a norma dell’articolo 13 della legge 7 Luglio 2009 n. 88. Gazzetta Ufficiale della Repubblica Italiana 2010, 106. [Google Scholar]
- Franus, W.; Franus, M.; Latosińska, J.; Wójcik, R. The use of spent glauconite in lightweight aggregate production. Boletín de la Sociedad Española de Cerámica y Vidrio. 2011, 50, 193–200. [Google Scholar]
- Shafigh, P.; Nomeli, M.A.; Johnson, A.U.; Bin, M.H.; Zamin, J.M. Engineering properties of lightweight aggregate concrete containing limestone powder and high volume fly ash. J. Clean. Prod. 2016, 135, 148–157. [Google Scholar] [CrossRef]
- Małgorzata, F.; Grzegorz, J.; Lidia, B.; Krzysztof, L.; Mieczysław, H.; Wojciech, F. Modification of lightweight aggregates’ microstructure by used motor oil addition. Materials 2016, 9, 845. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, second edition; Elsevier: London, UK, 2013. [Google Scholar]
- Farias, R.D.; Martínez, G.C.; Cotes, P.T.; Andreola, F.; Lancellotti, I.; Barbieri, L. Valorisation of agro-industrial waste in lightweight aggregates for agronomic use: Preliminary study. Environ. Eng. Manag. J. 2017, 16, 1691–1699. [Google Scholar] [CrossRef]
- European Parliament and European Council. Regulation (EC) No 2003/2003 of the European Parliament and of the Council Related to Fertilizers; European Union: Brussels, Belgium, 13 October 2003. [Google Scholar]
- Campos Vega, R.; Loarca-Pina, G.; Vergara-Castaneda, H.; Dave Oomah, B. Spent Coffee Grounds: A Review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Lubkowski, K.; Gzmil, B. Controlled release fertilizers. Pol. J. Chem. Technol. 2007, 9, 81–84. [Google Scholar] [CrossRef]




| C [wt.%] | H [wt.%] | N [wt.%] | S [wt.%] | O [wt.%] | ASH [wt.%] | HHVdry [MJ/kg] |
|---|---|---|---|---|---|---|
| 48.67 | 6.54 | 2.27 | 0 | 40.03 | 2.43 | 20.48 |
| Oxide [wt.%] | Red Clay | SCGs | FG |
|---|---|---|---|
| SiO2 | 52.77 | 0.37 | 31.85 |
| Al2O3 | 17.95 | 0.03 | 3.85 |
| Fe2O3 | 7.89 | 0.02 | 0.23 |
| MgO | 3.88 | 0.17 | 1.44 |
| K2O | 2.85 | 0.58 | 12.10 |
| CaO | 2.57 | 0.19 | 26.50 |
| TiO2 | 0.78 | 0.01 | 0.06 |
| Na2O | 0.66 | 0.14 | 6.19 |
| MnO | 0.19 | 0.00 | 0.00 |
| P2O5 | 0.00 | 0.32 | 17.50 |
| L.O.I (1050 °C) | 9.90 | 98.05 | 0.00 |
| TOTAL | 99.44 | 99.88 | 99.72 |
| MIX | W.A. [wt.%] (Boiling Water) | W.A. [wt.%] (Static Water) | pH | Specific Conductivity S.C. [mS/cm] | Real Density [g/cm3] | Bulk Density [g/cm3] | Total Porosity [%] |
|---|---|---|---|---|---|---|---|
| BS15 | 26.45 | 18.70 | 7 | 0.453 | 2.764 | 1.410 | 48.98 |
| SCG10 | 25.23 | 15.48 | 6.7 | 0.193 | 2.569 | 1.416 | 44.89 |
| SCG15 | 37.77 | 17.14 | 6.8 | 0.171 | 2.781 | 1.237 | 55.51 |
| SCG20 | 46.78 | 30.16 | 7.2 | 0.265 | 2.629 | 1.124 | 57.24 |
| SCG15FG10 | 20.90 | 15.09 | 6.7 | 0.28 | 2.73 | 1.34 | 50.88 |
| SCG15FG30 | 18.53 | 14.29 | 6.9 | 0.19 | 2.57 | 1.40 | 45.35 |
| SCG15FG50 | 15.88 | 12.04 | 6.8 | 0.17 | 2.49 | 1.41 | 43.63 |
| Elements [wt.% of Release] | 30 min | 21 days |
|---|---|---|
| Si | 0.17 | 7.59 |
| Al | 0.20 | 36.04 |
| Na | 0.00 | 29.54 |
| K | 0.07 | 25.74 |
| Ca | 0.64 | 78.54 |
| Mg | 0.84 | 37.39 |
| P | 0.91 | 87.73 |
| Fe | 0.05 | 11.77 |
| Pb | 0.35 | 44.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreola, F.; Borghi, A.; Pedrazzi, S.; Allesina, G.; Tartarini, P.; Lancellotti, I.; Barbieri, L. Spent Coffee Grounds in the Production of Lightweight Clay Ceramic Aggregates in View of Urban and Agricultural Sustainable Development. Materials 2019, 12, 3581. https://doi.org/10.3390/ma12213581
Andreola F, Borghi A, Pedrazzi S, Allesina G, Tartarini P, Lancellotti I, Barbieri L. Spent Coffee Grounds in the Production of Lightweight Clay Ceramic Aggregates in View of Urban and Agricultural Sustainable Development. Materials. 2019; 12(21):3581. https://doi.org/10.3390/ma12213581
Chicago/Turabian StyleAndreola, Fernanda, Alessandro Borghi, Simone Pedrazzi, Giulio Allesina, Paolo Tartarini, Isabella Lancellotti, and Luisa Barbieri. 2019. "Spent Coffee Grounds in the Production of Lightweight Clay Ceramic Aggregates in View of Urban and Agricultural Sustainable Development" Materials 12, no. 21: 3581. https://doi.org/10.3390/ma12213581