Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitosan/Essential Oils Film Preparation
2.3. Characterization
2.3.1. Qualitative Assessment
2.3.2. Antimicrobial Susceptibility
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Wettability
2.3.5. Swelling Degree
- M0—film mass before swelling (g)
- Ms—film mass after swelling (g)
2.3.6. Scanning Electron Microscope (SEM)
2.3.7. Tensile Properties
2.3.8. Statistical Analysis
3. Results and Discussion
3.1. Qualitative Assessment of the Chitosan Solution and Chitosan/Essential Oils Emulsions
3.2. Hydrogen Potential (pH) of the Chitosan Solution and Chitosan/Essential Oils Emulsions
3.3. In Vitro Antibacterial Activity of the Chitosan Solution and Chitosan/Essential Oils Emulsions
3.4. Qualitative Assessment of the Chitosan and Chitosan/Essential Oils Films
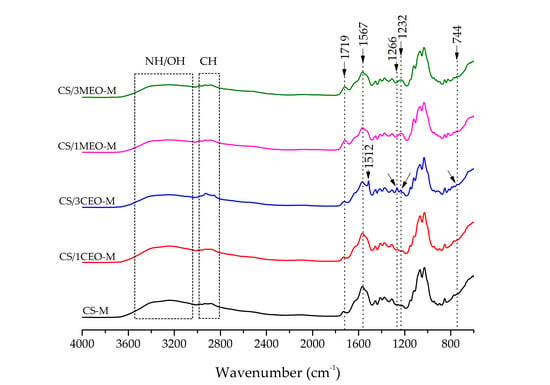
3.5. Fourier Transform Infrared Spectroscopy (FTIR) of the Chitosan and Chitosan/Essential Oils Films
3.6. Contact Angle Measurements of the Chitosan and Chitosan/Essential Oils Films
3.7. Swelling Test of the Chitosan and Chitosan/Essential Oils Films
3.8. Scanning Electron Microscopy (SEM) of the Chitosan and Chitosan/Essential Oils Films
3.9. Tensile Test of the Chitosan and Chitosan/Essential Oils Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, F.; Abdenour, A.; Bruno, M.; Silvia, P.; Alessandra, P.; Danilo, F.; Drifa, Y.-G.; Fahmi, E.M.; Khodir, M.; Mohamed, C. Chemical composition and in vitro antimicrobial, insecticidal and antioxidant activities of the essential oils of Mentha pulegium L. and Mentha rotundifolia (L.) Huds growing in Algeria. Ind. Crop. Prod. 2016, 88, 96–105. [Google Scholar] [CrossRef]
- Ocana-Fuentes, A.; Arranz-Gutierrez, E.; Senorans, F.; Reglero, G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: Anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem. Toxicol. 2010, 48, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason. Sonochem. 2016, 31, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-F.; Hsieh, C.-T.; Hsieh, T.-J.; Chang, F.-R.; Wang, C.-K. In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J. Food Drug Anal. 2015, 23, 124–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Anderson, R.A.; Zhan, Z.; Luo, R.; Guo, X.; Guo, Q.; Zhou, J.; Kong, J.; Davis, P.A.; Stoecker, B.J. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J. Tradit. Complementary Med. 2016, 6, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, D.C.; Costa, H.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Kim, J.-E.; Son, J.E.; Jeong, H.; Kim, D.J.; Seo, S.K.; Lee, E.; Lim, T.G.; Kim, J.R.; Chen, H.; Bode, A.M. A novel cinnamon-related natural product with Pim-1 inhibitory activity inhibits leukemia and skin cancer. Cancer Res. 2015, 75, 2716–2728. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G. Natural antimicrobials from plants. In New Methods of Food Preservation; Gould, G.W., Ed.; Springer: New York, NY, USA, 1995; pp. 58–89. [Google Scholar]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Antibacterial activity of different essential oils obtained from spices widely used in Mediterranean diet. Int. J. Food Sci. Technol. 2008, 43, 526–531. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz Navajas, Y.; Sánchez Zapata, E.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Chami, N.; Bennis, S.; Chami, F.; Aboussekhra, A.; Remmal, A. Study of anticandidal activity of carvacrol and eugenol in vitro and in vivo. Oral Microbiol. Immunol. 2005, 20, 106–111. [Google Scholar] [CrossRef]
- Zivanovic, S.; Chi, S.; Draughon, A.F. Antimicrobial activity of chitosan films enriched with essential oils. J. Food Sci. 2005, 70, M45–M51. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol. 2006, 44, 616–625. [Google Scholar] [CrossRef]
- Jose, A.V.; Ahmad, A.Z. Efficacy of alcohol-based and alcohol-free melaleuca oral solution for the treatment of fluconazole-refractory oropharyngeal candidiasis in patients with AIDS. HIV Clin. Trials 2002, 3, 379–385. [Google Scholar] [CrossRef]
- Bagg, J.; Jackson, M.S.; Sweeney, M.P.; Ramage, G.; Davies, A.N. Susceptibility to Melaleuca alternifolia (tea tree) oil of yeasts isolated from the mouths of patients with advanced cancer. Oral Oncol. 2006, 42, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Demurtas, C.; Piu, L. In vitro study on the anticandidal activity of Melaleuca alternifolia (tea tree) essential oil combined with chitosan. Flavour Fragr. J. 2008, 23, 227–231. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Misharina, T.; Samusenko, A. Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures. Appl. Biochem. Microbiol. 2008, 44, 438–442. [Google Scholar] [CrossRef]
- Yoshimura, M.; Amakura, Y.; Yoshida, T. Polyphenolic compounds in clove and pimento and their antioxidative activities. Biosci. Biotechnol. Biochem. 2011, 75, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dhamanigi, S.S.; Asad, M. Anti-stress activity of hydro-alcoholic extract of Eugenia caryophyllus buds (clove). Indian J. Pharmacol. 2009, 41, 28. [Google Scholar]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil–A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Lee, K.-G.; Shibamoto, T. Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]. Food Chem. 2001, 74, 443–448. [Google Scholar] [CrossRef]
- Matan, N.; Rimkeeree, H.; Mawson, A.; Chompreeda, P.; Haruthaithanasan, V.; Parker, M. Antimicrobial activity of cinnamon and clove oils under modified atmosphere conditions. Int. J. Food Microbiol. 2006, 107, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Oskoueian, E.; Maroufyan, E.; Goh, Y.; Ramezani-Fard, E.; Ebrahimi, M. Clove essential oil improves lipid peroxidation and antioxidant activity in tilapia fish fillet cooked by grilling and microwaving. World Acad. Sci. Eng. Technol. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2013, 7, 1176–1178. [Google Scholar]
- Ordonez, G.; Llopis, N.; Penalver, P. Efficacy of eugenol against a Salmonella enterica serovar enteritidis experimental infection in commercial layers in production. J. Appl. Poult. Res. 2008, 17, 376–382. [Google Scholar] [CrossRef]
- Martini, H.; Weidenborner, M.; Adams, S.; Kunz, B. Eugenol and carvacrol: The main fungicidal compounds in clove and savory. Ital. J. Food Sci. IJFS 1996, 8, 63–67. [Google Scholar]
- Cui, H.; Zhao, C.; Lin, L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control 2015, 56, 128–134. [Google Scholar] [CrossRef]
- Anwer, M.K.; Jamil, S.; Ibnouf, E.O.; Shakeel, F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J. Oleo Sci. 2014, 63, 347–354. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Khajeh, M.; Yamini, Y.; Bahramifar, N.; Sefidkon, F.; Pirmoradei, M.R. Comparison of essential oils compositions of Ferula assa-foetida obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem. 2005, 91, 639–644. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Riahi, L.; Chograni, H.; Elferchichi, M.; Zaouali, Y.; Zoghlami, N.; Mliki, A. Variations in Tunisian wormwood essential oil profiles and phenolic contents between leaves and flowers and their effects on antioxidant activities. Ind. Crop. Prod. 2013, 46, 290–296. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Souza, C.R.; Oliveira, W.P. Encapsulation of eugenol rich clove extract in solid lipid carriers. J. Food Eng. 2014, 127, 34–42. [Google Scholar] [CrossRef]
- Majeed, H.; Liu, F.; Hategekimana, J.; Sharif, H.R.; Qi, J.; Ali, B.; Bian, Y.-Y.; Ma, J.; Yokoyama, W.; Zhong, F. Bactericidal action mechanism of negatively charged food grade clove oil nanoemulsions. Food Chem. 2016, 197, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B Biointerfaces 2014, 114, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhou, H.; Lin, L. The specific antibacterial effect of the Salvia oil nanoliposomes against Staphylococcus aureus biofilms on milk container. Food Control 2016, 61, 92–98. [Google Scholar] [CrossRef]
- Hsieh, W.-C.; Chang, C.-P.; Gao, Y.-L. Controlled release properties of chitosan encapsulated volatile citronella oil microcapsules by thermal treatments. Colloids Surf. B Biointerfaces 2006, 53, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Vargas, M.; Atarés, L.; Chiralt, A. Physical properties of chitosan-basil essential oil edible films as affected by oil content and homogenization conditions. Procedia Food Sci. 2011, 1, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Amodio, M.L.; Colelli, G. Carvacrol-loaded chitosan nanoparticles maintain quality of fresh-cut carrots. Innov. Food Sci. Emerg. Technol. 2017, 41, 56–63. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.; Gavlighi, H.A.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; y Gómez, Y.G. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Turasan, H.; Sahin, S.; Sumnu, G. Encapsulation of rosemary essential oil. LWT Food Sci. Technol. 2015, 64, 112–119. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Schoukens, G. Bioactive dressings to promote wound healing. In Advanced Textiles for Wound Care, (2nd Edition); Rajendran, S., Ed.; Woodhead Publishing: Sawston/Cambridge, UK, 2019; pp. 135–167. [Google Scholar]
- Xu, H.; Ma, L.; Shi, H.; Gao, C.; Han, C. Chitosan–hyaluronic acid hybrid film as a novel wound dressing: In vitro and in vivo studies. Polym. Adv. Technol. 2007, 18, 869–875. [Google Scholar] [CrossRef]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of bioactive compounds-loaded chitosan films by using a QbD approach—A novel and potential wound dressing material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Lu, S.; Gao, W.; Gu, H.Y. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns J. Int. Soc. Burn Inj. 2008, 34, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci. 2000, 3, 303–311. [Google Scholar]
- Kumar, S.; Dutta, J.; Dutta, P. Preparation and characterization of N-heterocyclic chitosan derivative based gels for biomedical applications. Int. J. Biol. Macromol. 2009, 45, 330–337. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.; Mano, J. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef]
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin loaded gellan gum–chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Vinklárková, L.; Masteiková, R.; Foltýnová, G.; Muselík, J.; Pavloková, S.; Bernatonienė, J.; Vetchý, D. Film wound dressing with local anesthetic based on insoluble carboxymethycellulose matrix. J. Appl. Biomed. 2017, 15, 313–320. [Google Scholar] [CrossRef]
- Escárcega-Galaz, A.A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Sanches-Silva, A.; Madera-Santana, T.J.; Paseiro-Losada, P. Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings. Int. J. Biol. Macromol. 2018, 116, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef]
- Chin, C.-Y.; Jalil, J.; Ng, P.Y.; Ng, S.-F. Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application. J. Ethnopharmacol. 2018, 212, 188–199. [Google Scholar] [CrossRef]
- Il’Ina, A.; Varlamov, V. Hydrolysis of chitosan in lactic acid. Appl. Biochem. Microbiol. 2004, 40, 300–303. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch−chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Pal, S. Development of porous hydroxyapatite scaffolds. Mater. Manuf. Process. 2006, 21, 325–328. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência E Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Mason, T.; Wilking, J.; Meleson, K.; Chang, C.; Graves, S. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q. Rheological properties of chitosan–tripolyphosphate complexes: From suspensions to microgels. Carbohydr. Polym. 2012, 87, 1670–1677. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrieres, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Dos Santos Alves, K.; Vidal, R.R.L.; de Carvalho Balaban, R. Chitosan derivatives with thickening properties obtained by reductive alkylation. Mater. Sci. Eng. C 2009, 29, 641–646. [Google Scholar] [CrossRef]
- Luo, X.; Berlin, D.L.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. In situ generation of pH gradients in microfluidic devices for biofabrication of freestanding, semi-permeable chitosan membranes. Lab Chip 2010, 10, 59–65. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.-G.; Xue, Y.-P.; Liu, C.-S.; Yu, L.-J.; Ji, Q.-X.; Cha, D.S.; Park, H.J. Preparation and antibacterial activity of chitosan microshperes in a solid dispersing system. Front. Mater. Sci. China 2008, 2, 214–220. [Google Scholar] [CrossRef]
- Tsai, G.-J.; Su, W.-H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Aiedeh, K.; Taha, M.O. Synthesis of iron-crosslinked chitosan succinate and iron-crosslinked hydroxamated chitosan succinate and their in vitro evaluation as potential matrix materials for oral theophylline sustained-release beads. Eur. J. Pharm. Sci. 2001, 13, 159–168. [Google Scholar] [CrossRef]
- Papineau, A.M.; Hoover, D.G.; Knorr, D.; Farkas, D.F. Antimicrobial effect of water-soluble chitosans with high hydrostatic pressure. Food Biotechnol. 1991, 5, 45–57. [Google Scholar] [CrossRef]
- Sudarshan, N.; Hoover, D.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Gutha, Y.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Jiao, X. Antibacterial and wound healing properties of chitosan/poly (vinyl alcohol)/zinc oxide beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf. B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Nagori, B. A review on microorganisms causing wound infections on skin. Asian J. Pharm. Technol. 2013, 3, 119–122. [Google Scholar]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.; Duerden, B.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Jeon, S.J.; Oh, M.; Yeo, W.-S.; Galvao, K.N.; Jeong, K.C. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS ONE 2014, 9, e92723. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; El-Ghany, N.A.A. Synthesis and antimicrobial activity of some novel terephthaloyl thiourea cross-linked carboxymethyl chitosan hydrogels. Cellulose 2012, 19, 1879–1891. [Google Scholar] [CrossRef]
- Helander, I.; Nurmiaho-Lassila, E.-L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Huang, L.; Dai, T.; Xuan, Y.; Tegos, G.P.; Hamblin, M.R. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: Efficacy against bacterial burn infections. Antimicrob. Agents Chemother. 2011, 55, 3432–3438. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic properties of human skin and processed dermis. Ski. Res. Technol. 2001, 7, 18–23. [Google Scholar] [CrossRef]
- Smith, L.T.; Holbrook, K.A.; Byers, P.H. Structure of the dermal matrix during development and in the adult. J. Investig. Dermatol. 1982, 79, 93–104. [Google Scholar] [CrossRef]
- Ramshaw, J.A. Distribution of type III collagen in bovine skin of various ages. Connect. Tissue Res. 1986, 14, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Yasin, T. Controlled delivery of drug from pH sensitive chitosan/poly (vinyl alcohol) blend. Carbohydr. Polym. 2012, 88, 1055–1060. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Zivanovic, S.; Li, J.; Davidson, P.M.; Kit, K. Physical, mechanical, and antibacterial properties of chitosan/PEO blend films. Biomacromolecules 2007, 8, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.; Monte, M.L.; Pinto, L.A. Preparation of biopolymer film from chitosan modified with lipid fraction. Int. J. Food Sci. Technol. 2011, 46, 1856–1862. [Google Scholar] [CrossRef]
- Dilamian, M.; Montazer, M.; Masoumi, J. Antimicrobial electrospun membranes of chitosan/poly (ethylene oxide) incorporating poly (hexamethylene biguanide) hydrochloride. Carbohydr. Polym. 2013, 94, 364–371. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NY, USA, 2014. [Google Scholar]
- Chen, H.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of spray-dried zein–casein nanocapsules with co-encapsulated eugenol and thymol. J. Food Eng. 2015, 144, 93–102. [Google Scholar] [CrossRef]
- Monteiro, O.; Souza, A.; Soledade, L.; Queiroz, N.; Souza, A.; Mouchrek Filho, V.; Vasconcelos, A. Chemical evaluation and thermal analysis of the essential oil from the fruits of the vegetable species Pimenta dioica Lindl. J. Therm. Anal. Calorim. 2011, 106, 595–600. [Google Scholar] [CrossRef]
- Ravenelle, F.; Rahmouni, M. Contramid®: High-amylose starch for controlled drug delivery. Polysacch. Drug Deliv. Pharm. Appl. 2006, 934, 79–104. [Google Scholar]
- Tsai, H.-S.; Wang, Y.-Z. Properties of hydrophilic chitosan network membranes by introducing binary crosslink agents. Polym. Bull. 2008, 60, 103–113. [Google Scholar] [CrossRef]
- Zheng, Z.; Wei, Y.; Wang, G.; Ao, A.W.Q.; Gong, Y.; Zhang, X. Surface properties of chitosan films modified with polycations and their effects on the behavior of PC12 cells. J. Bioact. Compat. Polym. 2009, 24, 63–82. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Whu, S.W.; Tsai, C.-L.; Wu, Y.-H.; Chen, H.-W.; Hsieh, K.-H. Chitosan as scaffold materials: Effects of molecular weight and degree of deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar-Ricardo, A. Poly (vinyl alcohol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Membr. Sci. 2014, 469, 262–271. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Kitiyanant, Y. Chitosan scaffolds for in vitro buffalo embryonic stem-like cell culture: An approach to tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 80, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Thirawong, N.; Weerapol, Y.; Nunthanid, J.; Sungthongjeen, S. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur. J. Pharm. Biopharm. 2007, 67, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nimni, M.E.; Yang, Z.; Han, B. Chitosan/gelatin–based films crosslinked by proanthocyanidin. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 75, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen loaded PVA/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int. J. Pharm. 2010, 392, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, R.; Harding, I.; Bowater, I.; Christie, G.; Lonergan, G.T. Preparation, surface modification and characterisation of solution cast starch PVA blended films. Polym. Test. 2004, 23, 17–27. [Google Scholar] [CrossRef]
- Martins, C.S.; Morgado, D.L.; Assis, O.B.G. Cashew gum-chitosan blended films: Spectral, mechanical and surface wetting evaluations. Macromol. Res. 2016, 24, 691–697. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll. 2009, 23, 2102–2109. [Google Scholar] [CrossRef]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch–chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Elsner, J.J.; Shefy-Peleg, A.; Zilberman, M. Novel biodegradable composite wound dressings with controlled release of antibiotics: Microstructure, mechanical and physical properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, I.; Pakravan, M.; Rahimi, H.; Bahador, A.; Farshadzadeh, Z.; Haririan, I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 433–444. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A.; Coma, V. Effects of hydrophilic plasticizers on mechanical, thermal, and surface properties of chitosan films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.; Cortez-Rocha, M.; Ezquerra-Brauer, J.; Graciano-Verdugo, A.; Rodriguez-Félix, F.; Castillo-Ortega, M.; Yépiz-Gómez, M.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Wang, M.-Y.; West, B.J.; Jensen, C.J.; Nowicki, D.; Su, C.; Palu, A.K.; Anderson, G. Morinda citrifolia (Noni): A literature review and recent advances in Noni research. Acta Pharmacol. Sin. 2002, 23, 1127–1141. [Google Scholar]
- Hansen, B.; Jemec, G.B. The mechanical properties of skin in osteogenesis imperfecta. Arch. Dermatol. 2002, 138, 909–911. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Saraiva Sanchez, E.M.; da Costa, A.C.; Moraes, Â.M. The influence of preparation conditions on the characteristics of chitosan-alginate dressings for skin lesions. J. Appl. Polym. Sci. 2008, 109, 2703–2710. [Google Scholar] [CrossRef]
- Pimentel, T.A.; Durães, J.A.; Drummond, A.L.; Schlemmer, D.; Falcão, R.; Sales, M.J.A. Preparation and characterization of blends of recycled polystyrene with cassava starch. J. Mater. Sci. 2007, 42, 7530–7536. [Google Scholar] [CrossRef]
- Schlemmer, D.; Sales, M. Thermoplastic starch films with vegetable oils of Brazilian Cerrado: Thermal characterization. J. Therm. Anal. Calorim. 2009, 99, 675–679. [Google Scholar] [CrossRef]
- Pailler-Mattei, C.; Bec, S.; Zahouani, H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng. Phys. 2008, 30, 599–606. [Google Scholar] [CrossRef] [PubMed]




| Sample | pH | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Average | |
| CS-S | 2.60 | 2.62 | 2.65 | 2.62 A |
| CS/1CEO-E | 2.62 | 2.56 | 2.61 | 2.59 A |
| CS/3CEO-E | 2.55 | 2.60 | 2.60 | 2.58 A |
| CS/1MEO-E | 2.63 | 2.60 | 2.61 | 2.61 A |
| CS/3MEO-E | 2.63 | 2.63 | 2.56 | 2.61 A |
| Sample | Zone of Inhibition (mm) | ||
|---|---|---|---|
| Staphylococcus aureus Gram-Positive | Escherichia coli Gram-Negative | Candida albicans Fungi | |
| CEO | 36.0 | 16.0 | 21.0 |
| MEO | 9.0 | 10.0 | 12.0 |
| CS-S | 8.5 | 7.0 | 7.5 |
| CS/1CEO-E | 0.0 | 7.0 | 8.0 |
| CS/3CEO-E | 9.0 | 8.0 | 7.0 |
| CS/1MEO-E | 6.0 | 9.0 | 7.0 |
| CS/3MEO-E | 0.0 | 8.0 | 9.0 |
| Sample | Color | Homogeneity | Flexibility | Adhesion |
|---|---|---|---|---|
| CS-F | Transparent | High | High | High |
| CS/1CEO-F | Light yellow | Intermediary | Intermediary | Intermediary |
| CS/3CEO-F | Light yellow + | Low | Intermediary | Intermediary |
| CS/1MEO-F | Lightly yellowish | Intermediary | Intermediary | Intermediary |
| CS/3MEO-F | Lightly yellowish + | Low | Intermediary | Intermediary |
| Sample | Thickness (mm) |
|---|---|
| CS-F | 0.13 AB |
| CS/1CEO-F | 0.14 AB |
| CS/3CEO-F | 0.17 B |
| CS/1MEO-F | 0.11 A |
| CS/3MEO-F | 0.15 B |
| Sample | Contact Angle (°) | |
|---|---|---|
| Distilled Water (pH = 5.2) | PBS (pH = 7.2) | |
| Average | Average | |
| CS-M | 64.2 A | 67.7 A |
| CS/1CEO-M | 53.7 A | 62.8 A |
| CS/3CEO-M | 47.7 A | 58.3 A |
| CS/1MEO-M | 64.4 A | 56.9 A |
| CS/3MEO-M | 66.6 A | 65.8 A |
| Medium | Sample | Swelling (%) | ||
|---|---|---|---|---|
| 1 h | 2 h | 3 h | ||
| CS-F | - | - | - | |
| DW * (pH 5.5) | CS/1CEO-F | 678.57 A | 611.97 A | 971.04 A |
| CS/3CEO-F | - | - | - | |
| CS/1MEO-F | 2450.76 B | 2448.22 B | 3018.96 B | |
| CS/3MEO-F | 742.41 A | 961.21 A | 1227.62 A | |
| CS-F | - | - | - | |
| PBS (pH 6.4) | CS/1CEO-F | 3370.20 B | 886.87 A | 1149.14 A |
| CS/3CEO-F | 3565.60 B | 1364.64 A | 1536.49 A | |
| CS/1MEO-F | 5176.63 C | 3469.14 B | 4229.68 B | |
| CS/3MEO-F | 1336.1 7A | 999.91 A | 1161.00 A | |
| Sample | Maximum Stress (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| CS-F | 20.3 ± 4.5 | 43.0 ± 7.9 | 35.1 ± 6.6 |
| CS/1CEO-F | 5.2 ± 0.9 | 101.2 ± 5.8 | 5.4 ± 0.9 |
| CS/3CEO-F | 6.6 ± 1.7 | 105.1 ± 7.9 | 5.8 ± 1.2 |
| CS/1MEO-F | 11.7 ± 1.3 | 61.1 ± 3.8 | 32.3 ± 2.2 |
| CS/3MEO-F | 7.3 ± 0.4 | 53.6 ± 6.3 | 8.6 ± 1.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira dos Santos, E.; Nicácio, P.H.M.; Coêlho Barbosa, F.; Nunes da Silva, H.; Andrade, A.L.S.; Lia Fook, M.V.; de Lima Silva, S.M.; Farias Leite, I. Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties. Materials 2019, 12, 2223. https://doi.org/10.3390/ma12142223
Pereira dos Santos E, Nicácio PHM, Coêlho Barbosa F, Nunes da Silva H, Andrade ALS, Lia Fook MV, de Lima Silva SM, Farias Leite I. Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties. Materials. 2019; 12(14):2223. https://doi.org/10.3390/ma12142223
Chicago/Turabian StylePereira dos Santos, Elaine, Pedro Henrique Medeiros Nicácio, Francivandi Coêlho Barbosa, Henrique Nunes da Silva, André Luís Simões Andrade, Marcus Vinícius Lia Fook, Suédina Maria de Lima Silva, and Itamara Farias Leite. 2019. "Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties" Materials 12, no. 14: 2223. https://doi.org/10.3390/ma12142223