Blood Biomarkers and Metabolomic Profiling for the Early Diagnosis of Vancomycin-Associated Acute Kidney Injury: A Systematic Review and Meta-Analysis of Experimental Studies
Abstract
:1. Introduction
2. Material and Methods
2.1. Protocol and Registration
2.2. Inclusion and Exclusion Criteria
2.3. Outcomes of Interest
2.4. Search Strategy
2.5. Data Extraction
2.6. Assessment of Methodological Quality
2.7. Data Analysis and Synthesis
3. Results
3.1. Synthesis including All Data
3.2. Secondary Outcome
3.3. Risk of Bias, Quality of Evidence
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Sinha Ray, A.; Haikal, A.; Hammoud, K.A.; Yu, A.S. Vancomycin and the Risk of AKI: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 2132–2140. [Google Scholar] [CrossRef]
- Awdishu, L.; Le, A.; Amato, J.; Jani, V.; Bal, S.; Mills, R.H.; Carrillo-Terrazas, M.; Gonzalez, D.J.; Tolwani, A.; Acharya, A.; et al. Urinary Exosomes Identify Inflammatory Pathways in Vancomycin Associated Acute Kidney Injury. Int. J. Mol. Sci. 2021, 22, 2784. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Murray, K.P.; Lagnf, A.M.; Melvin, S.; Bhatia, S.; Shamim, M.D.; Smith, J.R.; Brade, K.D.; Simon, S.P.; Nagel, J.; et al. A Multicenter Evaluation of Vancomycin-Associated Acute Kidney Injury in Hospitalized Patients with Acute Bacterial Skin and Skin Structure Infections. Infect. Dis. Ther. 2020, 9, 89–106. [Google Scholar] [CrossRef]
- Lodise, T.P.; Patel, N.; Lomaestro, B.M.; Rodvold, K.A.; Drusano, G.L. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 2009, 49, 507–514. [Google Scholar] [CrossRef]
- Cano, E.L.; Haque, N.Z.; Welch, V.L.; Cely, C.M.; Peyrani, P.; Scerpella, E.G.; Ford, K.D.; Zervos, M.J.; Ramirez, J.A.; Kett, D.H.; et al. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: Retrospective analysis of the IMPACT-HAP Database. Clin. Ther. 2012, 34, 149–157. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Niederman, M.S.; Kollef, M.H.; Shorr, A.F.; Kunkel, M.J.; Baruch, A.; McGee, W.T.; Reisman, A.; Chastre, J. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A randomized, controlled study. Clin. Infect. Dis. 2012, 54, 621–629. [Google Scholar] [CrossRef]
- Kan, W.C.; Chen, Y.C.; Wu, V.C.; Shiao, C.C. Vancomycin-Associated Acute Kidney Injury: A Narrative Review from Pathophysiology to Clinical Application. Int. J. Mol. Sci. 2022, 23, 2052. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
- Qin, X.; Tsoi, M.F.; Zhao, X.; Zhang, L.; Qi, Z.; Cheung, B.M.Y. Vancomycin-associated acute kidney injury in Hong Kong in 2012–2016. BMC Nephrol. 2020, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- Pais, G.M.; Liu, J.; Zepcan, S.; Avedissian, S.N.; Rhodes, N.J.; Downes, K.J.; Moorthy, G.S.; Scheetz, M.H. Vancomycin-Induced Kidney Injury: Animal Models of Toxicodynamics, Mechanisms of Injury, Human Translation, and Potential Strategies for Prevention. Pharmacotherapy 2020, 40, 438–454. [Google Scholar] [CrossRef]
- Shah-Khan, F.; Scheetz, M.H.; Ghossein, C. Biopsy-Proven Acute Tubular Necrosis due to Vancomycin Toxicity. Int J. Nephrol. 2011, 2011, 436856. [Google Scholar] [CrossRef]
- Luque, Y.; Louis, K.; Jouanneau, C.; Placier, S.; Esteve, E.; Bazin, D.; Rondeau, E.; Letavernier, E.; Wolfromm, A.; Gosset, C.; et al. Vancomycin-Associated Cast Nephropathy. J. Am. Soc. Nephrol. JASN 2017, 28, 1723–1728. [Google Scholar] [CrossRef]
- Tantranont, N.; Luque, Y.; Hsiao, M.; Haute, C.; Gaber, L.; Barrios, R.; Adrogue, H.E.; Niasse, A.; Truong, L.D. Vancomycin-Associated Tubular Casts and Vancomycin Nephrotoxicity. Kidney Int. Rep. 2021, 6, 1912–1922. [Google Scholar] [CrossRef]
- Im, D.S.; Shin, H.J.; Yang, K.J.; Jung, S.Y.; Song, H.Y.; Hwang, H.S.; Gil, H.W. Cilastatin attenuates vancomycin-induced nephrotoxicity via P-glycoprotein. Toxicol. Lett. 2017, 277, 9–17. [Google Scholar] [CrossRef]
- Xu, W.; Mao, Z.; Zhao, B.; Ni, T.; Deng, S.; Yu, P.; He, J.; Mao, E. Vitamin C attenuates vancomycin induced nephrotoxicity through the reduction of oxidative stress and inflammation in HK-2 cells. Ann. Palliat. Med. 2021, 10, 1748–1754. [Google Scholar] [CrossRef]
- Xu, X.; Pan, J.; Li, H.; Li, X.; Fang, F.; Wu, D.; Zhou, Y.; Zheng, P.; Xiong, L.; Zhang, D. Atg7 mediates renal tubular cell apoptosis in vancomycin nephrotoxicity through activation of PKC-delta. FASEB J. 2019, 33, 4513–4524. [Google Scholar] [CrossRef]
- Du, H.; Li, Z.; Yang, Y.; Li, X.; Wei, Y.; Lin, Y.; Zhuang, X. New insights into the vancomycin-induced nephrotoxicity using in vitro metabolomics combined with physiologically based pharmacokinetic modeling. J. Appl. Toxicol. JAT 2020, 40, 897–907. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Hassan, S.M.; Sayed, A.M.; Abo-Youssef, A.M. Ameliorate impacts of scopoletin against vancomycin-induced intoxication in rat model through modulation of Keap1-Nrf2/HO-1 and IkappaBalpha-P65 NF-kappaB/P38 MAPK signaling pathways: Molecular study, molecular docking evidence and network pharmacology analysis. Int. Immunopharmacol. 2022, 102, 108382. [Google Scholar] [CrossRef]
- Hodoshima, N.; Masuda, S.; Inui, K. Decreased renal accumulation and toxicity of a new VCM formulation in rats with chronic renal failure. Drug Metab. Pharmacokinet. 2007, 22, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef]
- Wettersten, N.; Katz, R.; Shlipak, M.G.; Scherzer, R.; Waikar, S.S.; Ix, J.H.; Estrella, M.M. Urinary Biomarkers and Kidney Outcomes: Impact of Indexing Versus Adjusting for Urinary Creatinine. Kidney Med. 2021, 3, 546–554 e541. [Google Scholar] [CrossRef]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Oktem, F.; Arslan, M.K.; Ozguner, F.; Candir, O.; Yilmaz, H.R.; Ciris, M.; Uz, E. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: Protection by erdosteine. Toxicology 2005, 215, 227–233. [Google Scholar] [CrossRef]
- Naghibi, B.; Ghafghazi, T.; Hajhashemi, V.; Talebi, A.; Taheri, D. The effect of 2,3-dihydroxybenzoic acid and tempol in prevention of vancomycin-induced nephrotoxicity in rats. Toxicology 2007, 232, 192–199. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yano, T.; Hanada, Y.; Takeshita, A.; Inagaki, F.; Masuda, S.; Matsunaga, N.; Koyanagi, S.; Ohdo, S. Vancomycin induces reactive oxygen species-dependent apoptosis via mitochondrial cardiolipin peroxidation in renal tubular epithelial cells. Eur. J. Pharmacol. 2017, 800, 48–56. [Google Scholar] [CrossRef]
- Dalaklioglu, S.; Tekcan, M.; Gungor, N.E.; Celik-Ozenci, C.; Aksoy, N.H.; Baykal, A.; Tasatargil, A. Role of the poly(ADP-ribose)polymerase activity in vancomycin-induced renal injury. Toxicol. Lett. 2010, 192, 91–96. [Google Scholar] [CrossRef]
- Takigawa, M.; Masutomi, H.; Kishimoto, Y.; Shimazaki, Y.; Hamano, Y.; Kondo, Y.; Arai, T.; Lee, J.; Ishii, T.; Mori, Y.; et al. Time-Dependent Alterations of Vancomycin-Induced Nephrotoxicity in Mice. Biol. Pharm. Bull. 2017, 40, 975–983. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Lang, F.; Hu, L.; Tang, Q.; Wang, H.; Zhang, Y.; Hao, Z. Rutin Attenuates Vancomycin-Induced Nephrotoxicity by Ameliorating Oxidative Stress, Apoptosis, and Inflammation in Rats. Antimicrob. Agents Chemother. 2019, 63, e01545-18. [Google Scholar] [CrossRef] [Green Version]
- Avedissian, S.N.; Pais, G.; Liu, J.; O’Donnell, J.N.; Lodise, T.P.; Neely, M.; Prozialeck, W.C.; Lamar, P.C.; Becher, L.; Scheetz, M.H. The Pharmacodynamic-Toxicodynamic Relationship of AUC and C max in Vancomycin-Induced Kidney Injury in an Animal Model. Antimicrob. Agents Chemother. 2021, 65, e01945-20. [Google Scholar] [CrossRef]
- Bayram, A.; Erkan, G.N.; Talih, G.; Baskol, G.; Deniz, K.; Yildiz, K.; Esmaoglu, A. The alpha-2 receptor agonist dexmedetomidine attenuates vancomycininduced acute kidney injury. Bratisl. Lek. Listy 2019, 120, 429–433. [Google Scholar] [CrossRef]
- El Bohi, K.M.; Abdel-Motal, S.M.; Khalil, S.R.; Abd-Elaal, M.M.; Metwally, M.M.M.; WM, E.L. The efficiency of pomegranate (Punica granatum) peel ethanolic extract in attenuating the vancomycin-triggered liver and kidney tissues injury in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 7134–7150. [Google Scholar] [CrossRef]
- Cetin, H.; Olgar, S.; Oktem, F.; Ciris, M.; Uz, E.; Aslan, C.; Ozguner, F. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1181–1185. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Pais, G.M.; O’Donnell, J.N.; Lodise, T.P.; Liu, J.; Prozialeck, W.C.; Joshi, M.D.; Lamar, P.C.; Becher, L.; Gulati, A.; et al. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J. Antimicrob. Chemother. 2019, 74, 2326–2334. [Google Scholar] [CrossRef]
- Bruniera, F.R.; Ferreira, F.M.; Savioli, L.R.; Bacci, M.R.; Feder, D.; Pereira, E.C.; Pedreira, M.L.; Peterlini, M.A.; Perazzo, F.F.; Azzalis, L.A.; et al. Endothelial, renal and hepatic variables in Wistar rats treated with Vancomycin. An. Acad. Bras. Cienc. 2014, 86, 1963–1972. [Google Scholar] [CrossRef]
- Shi, H.H.; Guo, Y.; Chen, L.P.; Wang, C.C.; Huang, Q.R.; Xue, C.H.; Wang, Y.M.; Zhang, T.T. Docosahexaenoic Acid-Acylated Astaxanthin Esters Exhibit Superior Renal Protective Effect to Recombination of Astaxanthin with DHA via Alleviating Oxidative Stress Coupled with Apoptosis in Vancomycin-Treated Mice with Nephrotoxicity. Mar. Drugs 2021, 19, 499. [Google Scholar] [CrossRef]
- O’Donnell, J.N.; Rhodes, N.J.; Miglis, C.M.; Catovic, L.; Liu, J.; Cluff, C.; Pais, G.; Avedissian, S.; Joshi, M.D.; Griffin, B.; et al. Dose, duration, and animal sex predict vancomycin-associated acute kidney injury in preclinical studies. Int. J. Antimicrob. Agents 2018, 51, 239–243. [Google Scholar] [CrossRef]
- Dieterich, C.; Puey, A.; Lin, S.; Swezey, R.; Furimsky, A.; Fairchild, D.; Mirsalis, J.C.; Ng, H.H. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol. Sci. Off. J. Soc. Toxicol. 2009, 107, 258–269. [Google Scholar] [CrossRef]
- Chang, J.; Liu, J.; Kaye, K.S.; Scheetz, M.H. Vancomycin Duration of Therapy Can Inform the Need for Area Under the Curve Monitoring. Clin. Infect. Dis 2021, 73, e1235–e1236. [Google Scholar] [CrossRef]
- Chang, J.; Pais, G.M.; Valdez, K.; Marianski, S.; Barreto, E.F.; Scheetz, M.H. Glomerular Function and Urinary Biomarker Changes between Vancomycin and Vancomycin plus Piperacillin-Tazobactam in a Translational Rat Model. Antimicrob. Agents Chemother. 2022, 66, e0213221. [Google Scholar] [CrossRef] [PubMed]
- Barouxis, D.; Chalkias, A.; Syggelou, A.; Iacovidou, N.; Xanthos, T. Research in human resuscitation: What we learn from animals. J. Matern.-Fetal Neonatal Med. 2012, 25, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Liu, J.; Pais, G.M.; Chighine, A.; Kahnamoei, D.A.; Xanthos, T.; Chalkias, A.; Lee, A.; Hauser, A.R.; Chang, J.; et al. Urinary Metabolomics From a Dose-Fractionated Polymyxin B Rat Model of Acute Kidney Injury. Int. J. Antimicrob. Agents 2022, 60, 106593. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulos, I.; Boutsikos, I.; Mavrovounis, G.; Gkraikou, T.; Faa, G.; Barouxis, D.; Kesidou, E.; Mavridis, T.; Chalkias, A.; Xanthos, T. Stress hormones kinetics in ventricular fibrillation cardiac arrest and resuscitation: Translational and therapeutic implications. Am. J. Emerg Med. 2021, 50, 14–21. [Google Scholar] [CrossRef]
- Chalkias, A.; Spyropoulos, V.; Georgiou, G.; Laou, E.; Koutsovasilis, A.; Pantazopoulos, I.; Kolonia, K.; Vrakas, S.; Papalois, A.; Demeridou, S.; et al. Baseline Values and Kinetics of IL-6, Procalcitonin, and TNF-alpha in Landrace-Large White Swine Anesthetized with Propofol-Based Total Intravenous Anesthesia. Biomed. Res. Int 2021, 2021, 6672573. [Google Scholar] [CrossRef]
- Varvarousis, D.; Xanthos, T.; Ferino, G.; Noto, A.; Iacovidou, N.; Mura, M.; Scano, P.; Chalkias, A.; Papalois, A.; De-Giorgio, F.; et al. Metabolomics profiling reveals different patterns in an animal model of asphyxial and dysrhythmic cardiac arrest. Sci. Rep. 2017, 7, 16575. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.H.; Segar, J.L.; Teesch, L.M.; Kasper, D.C.; Schaefer, F.S.; Brophy, P.D. Urinary metabolomic markers of aminoglycoside nephrotoxicity in newborn rats. Pediatric Res. 2013, 73, 585–591. [Google Scholar] [CrossRef]
- Lenz, E.M.; Bright, J.; Knight, R.; Westwood, F.R.; Davies, D.; Major, H.; Wilson, I.D. Metabonomics with 1H-NMR spectroscopy and liquid chromatography-mass spectrometry applied to the investigation of metabolic changes caused by gentamicin-induced nephrotoxicity in the rat. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2005, 10, 173–187. [Google Scholar] [CrossRef]
- Xu, E.Y.; Perlina, A.; Vu, H.; Troth, S.P.; Brennan, R.J.; Aslamkhan, A.G.; Xu, Q. Integrated pathway analysis of rat urine metabolic profiles and kidney transcriptomic profiles to elucidate the systems toxicology of model nephrotoxicants. Chem. Res. Toxicol. 2008, 21, 1548–1561. [Google Scholar] [CrossRef]
- Cao, P.; Kang, Y.; Liu, J.; Liu, X.; Jin, Y.; Zhang, Z. Urinary metabolomics study of vancomycin-associated nephrotoxicity based on ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Hum. Exp. Toxicol. 2022, 41, 9603271221119178. [Google Scholar] [CrossRef]
- Tillman, L.; Tabish, T.A.; Kamaly, N.; Moss, P.; El-briri, A.; Thiemermann, C.; Pranjol, M.Z.I.; Yaqoob, M.M. Advancements in nanomedicines for the detection and treatment of diabetic kidney disease. Biomater. Biosyst. 2022, 6, 100047. [Google Scholar] [CrossRef]
- Eftekhari, A.; Maleki Dizaj, S.; Ahmadian, E.; Przekora, A.; Hosseiniyan Khatibi, S.M.; Ardalan, M.; Zununi Vahed, S.; Valiyeva, M.; Mehraliyeva, S.; Khalilov, R.; et al. Application of Advanced Nanomaterials for Kidney Failure Treatment and Regeneration. Mater. 2021, 14, 2939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Agborbesong, E.; Li, X. The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 1253. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Kuzovlev, A.; Noto, A.; d’Aloja, E.; Xanthos, T. Identifying the role of cytochrome c in post-resuscitation pathophysiology. Am. J. Emerg. Med. 2015, 33, 1826–1830. [Google Scholar] [CrossRef]
- Chalkias, A.; Fanos, V.; Noto, A.; Castren, M.; Gulati, A.; Svavarsdottir, H.; Iacovidou, N.; Xanthos, T. 1H NMR-metabolomics: Can they be a useful tool in our understanding of cardiac arrest? Resuscitation 2014, 85, 595–601. [Google Scholar] [CrossRef]
- Chalkias, A.; Xanthos, T. Redox-mediated programed death of myocardial cells after cardiac arrest and cardiopulmonary resuscitation. Redox Rep. Commun. Free Radic. Res. 2012, 17, 80–83. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, Z.; Zhu, J.; Zeng, M.; Liu, H.; Dong, Z. miR-214 represses mitofusin-2 to promote renal tubular apoptosis in ischemic acute kidney injury. Am. J. Physiol. Ren. Physiol. 2020, 318, F878–F887. [Google Scholar] [CrossRef]
- Brooks, C.; Wei, Q.; Cho, S.G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1alpha Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Tran, M.T.; Zsengeller, Z.K.; Berg, A.H.; Khankin, E.V.; Bhasin, M.K.; Kim, W.; Clish, C.B.; Stillman, I.E.; Karumanchi, S.A.; Rhee, E.P.; et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016, 531, 528–532. [Google Scholar] [CrossRef] [Green Version]



| Author Year | Species | VCM | Biomarker (Units) | Control | VCM Group | |||
|---|---|---|---|---|---|---|---|---|
| Administration | Dose (mg kg−1) | Frequency | Duration | |||||
| Oktem 2005 | Rats | Intraperitoneally | 200 | b.i.d. | 7 days | Creatinine (mg dL−1) | 0.44 ± 0.06 | 0.41 ± 0,05 |
| BUN (mg dL−1) | 18.8 ± 8.21 | 21.2 ± 5.1 | ||||||
| Naghibi 2007 | Rats | Intraperitoneally | 200 | b.i.d. | 7 days | Creatinine (mg dL−1) | ×2.5 | NA |
| BUN (mg dL−1) | ×5 | NA | ||||||
| Dalaklioglu 2010 | Rats | Intraperitoneally | 200 | b.i.d. | 7 days | Creatinine (mg dL−1) | 0.8 ± 0.04 | 3.38 ± 0.51 |
| BUN (mg dL−1) | 8.07 ± 0.75 | 53.87 ± 10.11 | ||||||
| Takigawa 2017 | Mice | Intraperitoneally | 400 | q.d. | 3 days | Creatinine (mg dL−1) | ×5.4 | NA |
| BUN (mg dL−1) | ×4.6 | NA | ||||||
| Mice | Intraperitoneally | 400 | q.d. | 5 days | Creatinine (mg dL−1) | NS | NA | |
| BUN (mg dL−1) | ×2.5 | NA | ||||||
| Mice | Intraperitoneally | 400 | q.d. | 7 days | Creatinine (mg dL−1) | ×4.0 | NA | |
| BUN (mg dL−1) | ×3.3 | NA | ||||||
| Qu 2018 | Rats | Intraperitoneally | 200 | b.i.d. | 7 days | Creatinine (mg dL−1) | N/A | NA |
| BUN (mg dL−1) | N/A | NA | ||||||
| Bayram 2019 | Rats | Intraperitoneally | 200 | b.i.d. | 7 days | Creatinine median (IQR) (mg dL−1) | 0.33 (0.29–0.34) | 0.52 (0.38–0.54) |
| BUN (mg dL−1) | 21.2 ± 3.5 | 35.18 ± 7.3 | ||||||
| El Bohi 2021 | Rats | Intraperitoneally | 443.6 | q.o.d. | 14 days | Creatinine (mg dL−1) | 0.83 ± 0.03 | 1.28 ± 0.1 |
| BUN (mg dL−1) | 27.75 ± 1.55 | 74.25 ± 2.14 | ||||||
| Uric acid (mg dL−1) | 6.16 ± 0.13 | 9.54 ± 0.23 | ||||||
| Cetin 2007 | Rats | Intraperitoneally | 200 | b.i.d. | 7 days | Creatinine (mg dL−1) | 0.43 ± 0.09 | 0.97 ± 0.19 |
| BUN (mg dL−1) | 16.25 ± 2.38 | 41.25 ± 4.13 | ||||||
| Bruniera 2014 | Rats | Intravenously | 10 (5 mg ml−1) | q.d. | 3 days | Creatinine (mg dL−1) | 0.5 ± 0.1 | 0.4 ± 0.1 |
| BUN (mg dL−1) | 49.0 ± 6.9 | 46.1 ± 6.0 | ||||||
| 10 (10 mg ml−1) | q.d. | 3 days | Creatinine (mg dL−1) | 0.5 ± 0.1 | 0.5 ± 0.1 | |||
| BUN (mg dL−1) | 49.0 ± 6.9 | 50.9 ± 9.0 | ||||||
| 10 (5 mg ml-1) | q.d. | 7 days | Creatinine (mg dL−1) | 0.6 ± 0.1 | 0.5 ± 0.1 | |||
| BUN (mg dL−1) | 50.9 ± 9.1 | 50.0 ± 3.8 | ||||||
| 10 (10 mg ml−1) | q.d. | 7 days | Creatinine (mg dL−1) | 0.6 ± 0.1 | 0.6 ± 0.1 | |||
| BUN (mg dL−1) | 50.9 ± 9.1 | 44.0 ± 4.8 | ||||||
| Shi 2021 | Mice | Intraperitoneally | 400 | q.d. | 3 days # | Creatinine (mg dL−1) | 69.2% | NA |
| BUN (mg dL−1) | 110% | NA | ||||||
| O’Donnell 2018 | Rat | Intraperitoneally | 150 | b.i.d. | 14 days | Creatinine (mg dL−1) | 365.6 ± 67.3 | 237.4 ± 23.7 |
| BUN (mg dL−1) | 26.8 ± 16.4 | 32.7 ± 3.5 | ||||||
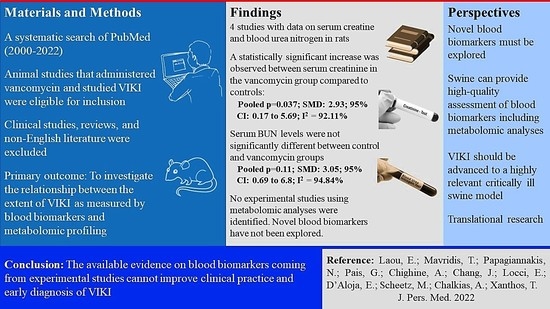
| Parameter | Number of Studies | N (Total) | Estimate (SMD) | p-Value | 95% CI | I2 | Q | p (Q) |
|---|---|---|---|---|---|---|---|---|
| Serum creatinine | 4 | 58 | 2.93 | 0.037 | 0.17 to 5.69 | 92.11% | 35.6 | <0.001 |
| Serum BUN | 3 | 46 | 3.05 | 0.11 | −0.69 to 6.80 | 94.84% | 23.16 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laou, E.; Mavridis, T.; Papagiannakis, N.; Pais, G.; Chighine, A.; Chang, J.; Locci, E.; D’Aloja, E.; Scheetz, M.; Chalkias, A.; et al. Blood Biomarkers and Metabolomic Profiling for the Early Diagnosis of Vancomycin-Associated Acute Kidney Injury: A Systematic Review and Meta-Analysis of Experimental Studies. J. Pers. Med. 2022, 12, 1397. https://doi.org/10.3390/jpm12091397
Laou E, Mavridis T, Papagiannakis N, Pais G, Chighine A, Chang J, Locci E, D’Aloja E, Scheetz M, Chalkias A, et al. Blood Biomarkers and Metabolomic Profiling for the Early Diagnosis of Vancomycin-Associated Acute Kidney Injury: A Systematic Review and Meta-Analysis of Experimental Studies. Journal of Personalized Medicine. 2022; 12(9):1397. https://doi.org/10.3390/jpm12091397
Chicago/Turabian StyleLaou, Eleni, Theodoros Mavridis, Nikolaos Papagiannakis, Gwendolyn Pais, Alberto Chighine, Jack Chang, Emanuela Locci, Ernesto D’Aloja, Marc Scheetz, Athanasios Chalkias, and et al. 2022. "Blood Biomarkers and Metabolomic Profiling for the Early Diagnosis of Vancomycin-Associated Acute Kidney Injury: A Systematic Review and Meta-Analysis of Experimental Studies" Journal of Personalized Medicine 12, no. 9: 1397. https://doi.org/10.3390/jpm12091397