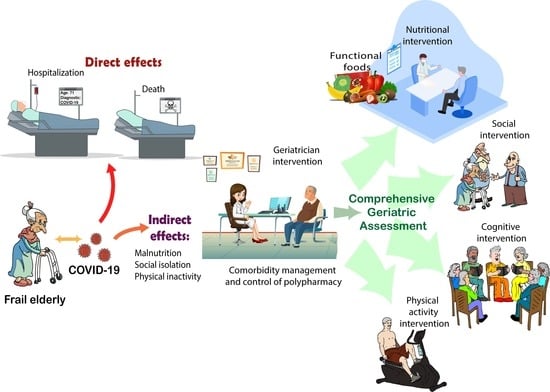
Direct and Indirect Effects of COVID-19 in Frail Elderly: Interventions and Recommendations
Abstract
:1. Introduction
2. Materials and Methods
Sources of the Data and Search Strategy
3. Frailty: A Vulnerability State
4. Frailty and COVID-19
4.1. Direct Effects of COVID-19 in Frail Population
4.2. Indirect Effects of COVID-19 in Frail Population
5. Interventions to Reduce Frailty
5.1. Evidence-Based Recommendations
Functional Foods
5.2. Experience-Based Interventions
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Patel, R.; Aggarwal, C.S.; Vunnam, R.; Sahu, N.; Bhatt, D.; et al. COVID-19 and older adults: What we know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef] [Green Version]
- Al-Zahrani, J. SARS-CoV-2 associated COVID-19 in geriatric population: A brief narrative review. Saudi J. Biol. Sci. 2020, 28, 738–743. [Google Scholar] [CrossRef]
- Lloyd-Sherlock, P.; Ebrahim, S.; Geffen, L.; McKee, M. Bearing the brunt of COVID-19: Older people in low and middle income countries. BMJ 2020, 368, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Maltese, G.; Corsonello, A.; Di Rosa, M.; Soraci, L.; Vitale, C.; Corica, F.; Lattanzio, F. Frailty and COVID-19: A systematic scoping review. J. Clin. Med. 2020, 9, 2106. [Google Scholar] [CrossRef] [PubMed]
- Woolford, S.J.; Sohan, O.; Dennison, E.M.; Cooper, C.; Patel, H.P. Approaches to the diagnosis and prevention of frailty. Aging Clin. Exp. Res. 2020, 32, 1629–1637. [Google Scholar] [CrossRef]
- Pilotto, A.; Custodero, C.; Maggi, S.; Polidori, M.C.; Veronese, N.; Ferrucci, L. A multidimensional approach to frailty in older people. Ageing Res. Rev. 2020, 60, 101047. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, 146–157. [Google Scholar] [CrossRef]
- Rockwood, K. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and comprehensive geriatric assessment. J. Korean Med. Sci. 2020, 35, 1–13. [Google Scholar] [CrossRef]
- Poco, P.C.E.; Aliberti, M.J.R.; Dias, M.B.; de Fatima Takahashi, S.; Leonel, F.C.; Altona, M.; Venys, A.L.; Shin-Ike, I.A.; Garcia, B.A.; Sumita, L.H.; et al. Divergent: Age, frailty, and atypical presentations of COVID-19 in hospitalized patients. J. Gerontol. Ser. A 2021, 76, e46–e51. [Google Scholar] [CrossRef]
- Kundi, H.; Çetin, E.H.Ö.; Canpolat, U.; Aras, S.; Celik, O.; Ata, N.; Birinci, S.; Çay, S.; Özeke, Ö.; Tanboğa, I.H.; et al. The role of frailty on adverse outcomes among older patients with COVID-19. J. Infect. 2020, 81, 944–951. [Google Scholar] [CrossRef]
- Lim, J.P.; Low, K.Y.H.; Lin, N.J.J.; Lim, C.Z.Q.; Ong, S.W.X.; Tan, W.Y.T.; Tay, W.C.; Tan, H.N.; Young, B.E.; Boon, D.L.C.; et al. Predictors for development of critical illness amongst older adults with COVID-19: Beyond age to age-associated factors. Arch. Gerontol. Geriatr. 2020, 94, 104331. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Vellas, C.; Delobel, P.; De Souto Barreto, P.; Izopet, J. COVID-19, virology and geroscience: A perspective. J. Nutr. Health Aging 2020, 24, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Larrañaga, A.; Dekhtyar, S.; Vetrano, D.L.; Bellander, T.; Fratiglioni, L. COVID-19: Risk accumulation among biologically and socially vulnerable older populations. Ageing Res. Rev. 2020, 63, 101149. [Google Scholar] [CrossRef] [PubMed]
- Woolford, S.J.; D’Angelo, S.; Curtis, E.M.; Parsons, C.M.; Ward, K.A.; Dennison, E.M.; Patel, H.P.; Cooper, C.; Harvey, N.C. COVID-19 and associations with frailty and multimorbidity: A prospective analysis of UK Biobank participants. Aging Clin. Exp. Res. 2020, 32, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hou, L.; Yang, X.; Huang, Z.; Yang, X.; Zhao, N.; He, M.; Shi, Y.; Kang, Y.; Yue, J.; et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: A prospective cohort study. BMC Med. 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Woodrow, L.; Ogliari, G.; Harwood, R. Association of frailty with mortality in older inpatients with COVID-19: A cohort study. Age Ageing 2020, 49, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Labenz, C.; Kremer, W.M.; Schattenberg, J.M.; Wörns, M.A.; Toenges, G.; Weinmann, A.; Galle, P.R.; Sprinzl, M.F. Clinical Frailty Scale for risk stratification in patients with SARS-CoV-2 infection. J. Investig. Med. 2020, 68, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Hägg, S.; Jylhävä, J.; Wang, Y.; Xu, H.; Metzner, C.; Annetorp, M.; Garcia-Ptacek, S.; Khedri, M.; Boström, A.-M.; Kadir, A.; et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J. Am. Med. Dir. Assoc. 2020, 21, 1555–1559. [Google Scholar] [CrossRef]
- Khan, K.T.; Hemati, K.; Donovan, A.L. Geriatric physiology and the frailty syndrome. Anesthesiol. Clin. 2019, 37, 453–474. [Google Scholar] [CrossRef]
- Walston, J.; Buta, B.; Xue, Q.L. Frailty screening and interventions: Considerations for clinical practice. Clin. Geriatr. Med. 2018, 34, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in older persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin. Geriatr. Med. 2011, 27, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- De Smet, R.; Mellaerts, B.; Vandewinckele, H.; Lybeert, P.; Frans, E.; Ombelet, S.; Lemahieu, W.; Symons, R.; Ho, E.; Frans, J.; et al. Frailty and mortality in hospitalized older adults with covid-19: Retrospective observational study. J. Am. Med. Dir. Assoc. 2020, 21, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Henrina, J.; Lim, M.A.; Lawrensia, S.; Yonas, E.; Vania, R.; Huang, I.; Lukito, A.A.; Suastika, K.; Kuswardhani, R.A.T.; et al. Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis. Arch. Gerontol. Geriatr. 2021, 93, 104324. [Google Scholar] [CrossRef]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef] [Green Version]
- Steinmeyer, Z.; Vienne-Noyes, S.; Piau, A.; Sourdet, S.; Bernard, M.; Steinmeyer, A.; Balardy, L. Acute care of older patients with COVID-19: Clinical characteristics and outcomes. Geriatrics 2020, 5, 65. [Google Scholar] [CrossRef]
- Knights, H.; Mayor, N.; Millar, K.; Cox, M.; Bunova, E.; Hughes, M.; Baker, J.; Mathew, S.; Russell-Jones, D.; Kotwica, A. Characteristics and outcomes of patients with COVID-19 at a district general hospital in Surrey, UK. Clin. Med. 2020, 20, E148–E153. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.T.; Short, R.; Carter, B.; Verduri, A.; Myint, P.K.; Quinn, T.J.; Vilches-Moraga, A.; Stechman, M.J.; Moug, S.; McCarthy, K.; et al. The clinical frailty scale: Estimating the prevalence of frailty in older patients hospitalised with COVID-19. The COPE Study. Geriatrics 2020, 5, 58. [Google Scholar] [CrossRef]
- Tehrani, S.; Killander, A.; Åstrand, P.; Jakobsson, J.; Gille-Johnson, P. Risk factors for death in adult COVID-19 patients: Frailty predicts fatal outcome in older patients. Int. J. Infect. Dis. 2021, 102, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Hanlon, P.; Gray, S.R.; Welsh, P.; Gill, J.M.R.; Foster, H.; Katikireddi, S.V.; Lyall, D.; Mackay, D.F.; O’Donnell, C.A.; et al. Comparison of two different frailty measurements and risk of hospitalisation or death from COVID-19: Findings from UK Biobank. BMC Med. 2020, 18, 355. [Google Scholar] [CrossRef]
- Bielza, R.; Sanz, J.; Zambrana, F.; Arias, E.; Malmierca, E.; Portillo, L.; Thuissard, I.J.; Lung, A.; Neira, M.; Moral, M.; et al. Clinical characteristics, frailty and mortality of residents with COVID-19 in nursing homes of a region of Madrid. J. Am. Med. Dir. Assoc. 2020, 245–252. [Google Scholar] [CrossRef]
- Martin, P.J.; Billet, S.; Landkocz, Y.; Fougère, B. Inflammation at the crossroads: The combined effects of COVID-19, ageing, and air pollution. J. Frailty Aging 2021, 1–5. [Google Scholar] [CrossRef]
- Meftahi, G.H.; Jangravi, Z.; Sahraei, H.; Bahari, Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: The contribution of “inflame-aging”. Inflamm. Res. 2020, 69, 825–839. [Google Scholar] [CrossRef]
- Shenoy, S. Coronavirus (Covid-19) sepsis: Revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 2020, 69, 1077–1085. [Google Scholar] [CrossRef]
- Sablerolles, R.S.G.; Lafeber, M.; van Kempen, J.A.L.; van de Loo, B.P.A.; Boersma, E.; Rietdijk, W.J.R.; Polinder-Bos, H.A.; Mooijaart, S.P.; van der Kuy, H.; Versmissen, J.; et al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): An international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev. 2021, 2, e163–e170. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S.; Thiruchelvam, K.; Aldeyab, M. Association of frailty and mortality in patients with COVID-19: A meta-analysis. Br. J. Anaesth. 2020, e108–e110. [Google Scholar] [CrossRef]
- Bellelli, G.; Rebora, P.; Valsecchi, M.G.; Bonfanti, P.; Citerio, G.; Galimberti, S.; Blangiardo, P.; Celora, G.M.; Deiana, V.; Ghezzi, N.; et al. Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Med. 2020, 46, 1634–1636. [Google Scholar] [CrossRef]
- Owen, R.K.; Conroy, S.P.; Taub, N.; Jones, W.; Bryden, D.; Pareek, M.; Faull, C.; Abrams, K.R.; Davis, D.; Banerjee, J. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: A retrospective observational study using electronic health records. Age Ageing 2021, 50, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Ogedengbe, O.; Agarwal, P.; Money-Coomes, S.; Abdurrahman, A.Z.; Mohammed, S.; Kalra, P.A.; Rothwell, N.; Pradhan, S. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting- a cohort study. BMC Geriatr. 2020, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Donato-Woodger, S.; Dainton, C.J. Competing crises: COVID-19 countermeasures and social isolation among older adults in long-term care. J. Adv. Nurs. 2020, 76, 2456–2459. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Rolland, Y. The importance of physical activity to care for frail older adults during the COVID-19 pandemic. J. Am. Med. Dir. Assoc. 2020, 21, 973–976. [Google Scholar] [CrossRef]
- Gorenko, J.A.; Moran, C.; Flynn, M.; Dobson, K.; Konnert, C. Social isolation and psychological distress among older adults related to COVID-19: A narrative review of remotely-delivered interventions and decommendations. J. Appl. Gerontol. 2021, 40, 3–13. [Google Scholar] [CrossRef]
- Chen, A.T.; Ge, S.; Cho, S.; Teng, A.K.; Chu, F.; Demiris, G.; Zaslavsky, O. Reactions to COVID-19, information and technology use, and social connectedness among older adults with pre-frailty and frailty. Geriatr. Nurs. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical frailty: ICFSR International clinical practice guidelines for identification and management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef] [Green Version]
- Bergman, Y.S.; Cohen-Fridel, S.; Shrira, A.; Bodner, E.; Palgi, Y. COVID-19 health worries and anxiety symptoms among older adults: The moderating role of ageism. Int. Psychogeriatr. 2020, 32, 1371–1375. [Google Scholar] [CrossRef]
- Brooke, J.; Clark, M. Older people’s early experience of household isolation and social distancing during COVID-19. J. Clin. Nurs. 2020, 29, 4387–4402. [Google Scholar] [CrossRef]
- Andrew, M.K.; Searle, S.D.; McElhaney, J.E.; McNeil, S.A.; Clarke, B.; Rockwood, K.; Kelvin, D.J. COVID-19, frailty and long-term care: Implications for policy and practice. J. Infect. Dev. Ctries. 2020, 14, 428–432. [Google Scholar] [CrossRef]
- Lidoriki, I.; Frountzas, M.; Schizas, D. Could nutritional and functional status serve as prognostic factors for COVID-19 in the elderly? Med. Hypotheses 2020, 144, 109946. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Saporiti, E.; Proietti, M.; Cesari, M. Nutritional considerations in frail older patients with COVID-19. J. Nutr. Health Aging 2020, 24, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Otobe, Y.; Suzuki, M.; Koyama, S.; Kikuchi, T.; Kusumi, H.; Arai, H. Effect of the COVID-19 epidemic on physical activity in community-dwelling older adults in Japan: A cross-sectional online survey. J. Nutr. Health Aging 2020, 24, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Roschel, H.; Artioli, G.G.; Gualano, B. Risk of increased physical inactivity during COVID-19 outbreak in older people: A call for actions. J. Am. Geriatr. Soc. 2020, 68, 1126–1128. [Google Scholar] [CrossRef]
- Farrell, T.W.; Francis, L.; Brown, T.; Ferrante, L.E.; Widera, E.; Rhodes, R.; Rosen, T.; Hwang, U.; Witt, L.J.; Thothala, N.; et al. Rationing limited healthcare resources in the COVID-19 era and beyond: Ethical considerations regarding older adults. J. Am. Geriatr. Soc. 2020, 68, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.G.; Dent, E.; Morley, J.E.; Merchant, R.A.; Beilby, J.; Beard, J.; Tripathy, C.; Sorin, M.; Andrieu, S.; Aprahamian, I.; et al. Screening for and managing the person with frailty in primary care: ICFSR consensus guidelines. J. Nutr. Health Aging 2020, 24, 920–927. [Google Scholar] [CrossRef]
- Puts, M.T.E.; Toubasi, S.; Andrew, M.K.; Ashe, M.C.; Ploeg, J.; Atkinson, E.; Ayala, A.P.; Roy, A.; Monforte, M.R.; Bergman, H.; et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: A scoping review of the literature and international policies. Age Ageing 2017, 46, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Turner, G.; Clegg, A. Best practice guidelines for the management of frailty: A British geriatrics society, age UK and royal college of general practitioners report. Age Ageing 2014, 43, 744–747. [Google Scholar] [CrossRef] [Green Version]
- Marcucci, M.; Damanti, S.; Germini, F.; Apostolo, J.; Bobrowicz-Campos, E.; Gwyther, H.; Holland, C.; Kurpas, D.; Bujnowska-Fedak, M.; Szwamel, K.; et al. Interventions to prevent, delay or reverse frailty in older people: A journey towards clinical guidelines. BMC Med. 2019, 17, 193. [Google Scholar] [CrossRef] [Green Version]
- Gale, C.R.; Westbury, L.; Cooper, C. Social isolation and loneliness as risk factors for the progression of frailty: The english longitudinal study of ageing. Age Ageing 2018, 47, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Freer, K.; Wallington, S.L. Social frailty: The importance of social and environmental factors in predicting frailty in older adults. Br. J. Community Nurs. 2019, 24, 486–492. [Google Scholar] [CrossRef]
- Kim, A.; Yi, E.; Kim, J.; Kim, M. A study on the influence of social leisure activities on the progression to the stage of frailty in Korean seniors. Int. J. Environ. Res. Public Health 2020, 17, 8909. [Google Scholar] [CrossRef]
- Granbom, M.; Kristensson, J.; Sandberg, M. Effects on leisure activities and social participation of a case management intervention for frail older people living at home: A randomised controlled trial. Health Soc. Care Community 2017, 25, 1416–1429. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.P.; Feng, L.; Nyunt, M.S.Z.; Feng, L.; Niti, M.; Tan, B.Y.; Chan, G.; Khoo, S.A.; Chan, S.M.; Yap, P.; et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. Am. J. Med. 2015, 128, 1225–1236.e1. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, L.J.; Barbagallo, M. The relevance of nutrition for the concept of cognitive frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 61–68. [Google Scholar] [CrossRef]
- Annweiler, G.; Beaudenon, M.; Gautier, J.; Simon, R.; Dubée, V.; Gonsard, J.; Parot-Schinkel, E.; Aidoud, A.; Albaret, G.; Annweiler, C.; et al. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): Study protocol for a randomized controlled trial. Trials 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Lorenzo-López, L.; Maseda, A.; De Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paddon-Jones, D.; Short, K.R.; Campbell, W.W.; Volpi, E.; Wolfe, R.R. Role of dietary protein in the sarcopenia of aging. Am. J. Clin. Nutr. 2008, 87, 1562–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Woo, J. Nutritional interventions to prevent and treat frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, J.E.; Hwang, H.S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef]
- Lozano-Montoya, I.; Correa-Pérez, A.; Abraha, I.; Soiza, R.L.; Cherubini, A.; O’Mahony, D.; Cruz-Jentoft, A.J. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: A systematic overview—The SENATOR Project ONTOP Series. Clin. Interv. Aging 2017, 12, 721–740. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.D.; Chen, H.C.; Huang, S.W.; Liou, T.H. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: A systematic review and meta-regression analysis of randomized trials. Nutrients 2019, 11, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labra, C.D.; Guimaraes-Pinheiro, C.; Maseda, A.; Lorenzo, T.; Millán-Calenti, J.C. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2015, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.S.; Pinheiro, M.B.; Fairhall, N.; Walsh, S.; Franks, T.C.; Kwok, W.; Bauman, A.; Sherrington, C. Evidence on physical activity and the prevention of frailty and sarcopenia among older people: A systematic review to inform the world health organization physical activity guidelines. J. Phys. Act. Health 2020, 17, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Negm, A.M.; Kennedy, C.C.; Thabane, L.; Veroniki, A.A.; Adachi, J.D.; Richardson, J.; Cameron, I.D.; Giangregorio, A.; Petropoulou, M.; Alsaad, S.M.; et al. Management of frailty: A systematic review and network meta-analysis of randomized controlled trials. J. Am. Med. Dir. Assoc. 2019, 20, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, D.; Polamarasetti, P. Nutrition planning during the COVID-19 pandemic for aging immunity. Bioact. Compd. Health Dis. 2020, 3, 109. [Google Scholar] [CrossRef]
- Singh, P.; Tripathi, M.K.; Yasir, M.; Khare, R.; Tripathi, M.K.; Shrivastava, R. Potential inhibitors for SARS-CoV-2 and functional food components as nutritional supplement for COVID-19: A review. Plant Foods Hum. Nutr. 2020, 75, 458–466. [Google Scholar] [CrossRef]
- Haslberger, A.; Jacob, U.; Hippe, B.; Karlic, H. Mechanisms of selected functional foods against viral infections with a view on COVID-19: Mini review. Funct. Foods Health Dis. 2020, 10, 195. [Google Scholar] [CrossRef]
- Alkhatib, A. Antiviral functional foods and exercise lifestyle prevention of coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef]
- Bandyopadhyay, P. Role of functional foods in COVID-19 situation. Int. Res. J. Mod. Eng. Technol. Sci. 2021, 3, 2582–5208. [Google Scholar]
- Hamid, H.; Thakur, A.; Thakur, N.S. Role of functional food components in COVID-19 pandemic: A review. Ann. Phytomed. Int. J. 2021, 10. [Google Scholar] [CrossRef]
- Moscatelli, F.; Sessa, F.; Valenzano, A.; Polito, R.; Monda, V.; Cibelli, G.; Villano, I.; Pisanelli, D.; Perrella, M.; Daniele, A.; et al. Covid-19: Role of nutrition and supplementation. Nutrients 2021, 13, 976. [Google Scholar] [CrossRef]
- Rastmanesh, R.; Marotta, F.; Tekin, I. Call for mobilization of functional foods, antioxidants, and herbal antivirals in support of international campaign to control coronavirus. Bioact. Compd. Health Dis. 2020, 3, 90–94. [Google Scholar] [CrossRef]
- Di Matteo, G.; Spano, M.; Grosso, M.; Salvo, A.; Ingallina, C.; Russo, M.; Ritieni, A.; Mannina, L. Food and COVID-19: Preventive/co-therapeutic strategies explored by current clinical trials and in silico studies. Foods 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Rakib, A.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Shahid-Ud-daula, A.F.M.; Amin, M.N.; Simal-Gandara, J. The genus curcuma and inflammation: Overview of the pharmacological perspectives. Plants 2021, 10, 63. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; My, T.T.A.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Loan, H.T.P.; Triet, N.T.; van Anh, T.T.; Quy, P.T.; van Tat, P.; et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega 2020, 5, 8312–8320. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]




| Model | Clinical Characteristics | References |
|---|---|---|
| Physical phenotype | Weight loss and Sarcopenia Weakness Exhaustation self-report Slow walking speed Low physical activity | Fried et al. [8] |
| Accumulation of clinical deficits | Detrimental to health conditions Age-related Failure of multiple physiological systems | Rockwood [9] |
| Study Type | Frailty Prevalence (%) | Average Age (Years) | Residential Status | Comorbidities | Geriatric Syndromes | Ref. |
|---|---|---|---|---|---|---|
| Retrospective cohort | 10% | 85 | Community | Hypertension 69.1%, Cardiac disease 48.9%, Dementia 45.7%, Respiratory disease 37.2%, Diabetes mellitus 11.7% | ADL dependency 64.9%, IADL dependency 76.1%, Polypharmacy 69.1%, Malnutrition 44.7% | [30] |
| Cross-sectional | 14% | 81 | NI | Hypertension 58%, Diabetes mellitus 31%, COPD 19%, Dementia 15%, Chronic kidney disease 14% | NI | [21] |
| Cross-sectional | 50% markers frailty | 68.7 | Community and home residents | Hypertension 45%, obesity 31%, diabetes 23%, dementia 15% | Polypharmacy 30%, Mobility aids 10%, Cognitive impairment 19.4%, Delirium 21%, Falls 8% | [31] |
| Cross-sectional | 67.4% (HFRS>5) | 74.1 | Community | Hypertension 78.8%, CAD 39.3%, Diabetes mellitus 36.2%, COPD 35.9%, Iron deficiency anemia 27.2%, Cerebrovascular disease 18.9%, Renal failure 8.8%, Depression 21.8%, Cancer 7.8%. | NI | [12] |
| Cross-sectional | 66.9% (CFS > 5) | 79.9 | Community | Diabetes 28%, CAD 26.9%, Hypertension 56.1%, COPD 14.5%, Heart failure 12.6% | NI | [32] |
| Retrospective, observational | ND | 59 | Community | Diabetes mellitus 22.5% Hypertension 38.1% Hyperlipidaemia 44.7% CAD 12.3% CKD 2.9% COPD 6.1% | Polypharmacy 26.5% Chronic pain 7.6% Memory problems 2.5% Nutritional risk 6.9% | [13] |
| Retrospective cohort | 74% | 66 | Community | Hypertension 54% Diabetes 31% CKD 19% CAD 13% Stroke 9% COPD 5% Dementia 6% Cancer 5% | NI | [33] |
| Frailty Model | Age (Years) | Participants | Category | Clinical Outcome | Study Design | Ref. |
|---|---|---|---|---|---|---|
| Fried phenotype FI | 37–73 | 802 | Frail and pre-frail | Increased in severity of disease for both models | Cohort multicentric | [34] |
| CFS | 65–97 | 81 | CFS > 7 | No survivors were frailer | Restrospective, single-center observational | [27] |
| CSF | 82–91 | 289 in hospital 341 in nursing homes | CFS >6 | Significantly associated with mortality after 30 days | Retrospective, observational, longitudinal | [35] |
| Frail Non-Disabled survey | 62–99 | 94 | Frail | No correlated with mortality | Retrospective cohort study | [30] |
| CFS HFRS | Median age 81 | 967 (250 patients with COVID-19) | CFS > 5 | Associated with in-hospital mortality and decreased probability of being discharge. No HFRS relationship found | Cross-sectional single center | [21] |
| CFS | 54–72 | 42 | Higher CFS scores CFS < 3 | Higher risk of mechanical ventilation. Correlated with earlier and more frequently discharge from home | Retrospective cohort study, single center | [20] |
| HFRS | Mean age 74.1 | 18,234 | >5 points | Correlated with all-cause in-hospital mortality, long stay (more than ten days) and use of mechanical ventilation | Cross-sectional, multicenter | [12] |
| FRAIL | 60–96 | 114 | Frail vs. no frail | Association with severe disease | Prospective cohort study | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizano-Escalante, M.G.; Anaya-Esparza, L.M.; Nuño, K.; Rodríguez-Romero, J.d.J.; Gonzalez-Torres, S.; López-de la Mora, D.A.; Villagrán, Z. Direct and Indirect Effects of COVID-19 in Frail Elderly: Interventions and Recommendations. J. Pers. Med. 2021, 11, 999. https://doi.org/10.3390/jpm11100999
Pizano-Escalante MG, Anaya-Esparza LM, Nuño K, Rodríguez-Romero JdJ, Gonzalez-Torres S, López-de la Mora DA, Villagrán Z. Direct and Indirect Effects of COVID-19 in Frail Elderly: Interventions and Recommendations. Journal of Personalized Medicine. 2021; 11(10):999. https://doi.org/10.3390/jpm11100999
Chicago/Turabian StylePizano-Escalante, María Guadalupe, Luis Miguel Anaya-Esparza, Karla Nuño, José de Jesús Rodríguez-Romero, Sughey Gonzalez-Torres, David A. López-de la Mora, and Zuamí Villagrán. 2021. "Direct and Indirect Effects of COVID-19 in Frail Elderly: Interventions and Recommendations" Journal of Personalized Medicine 11, no. 10: 999. https://doi.org/10.3390/jpm11100999