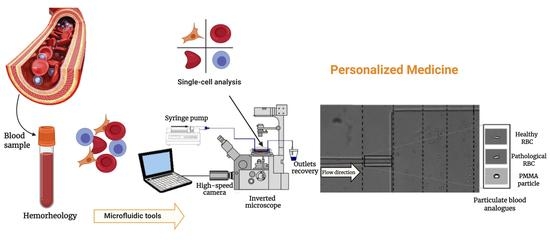
Visualization and Measurements of Blood Cells Flowing in Microfluidic Systems and Blood Rheology: A Personalized Medicine Perspective
Abstract
:1. Introduction
2. Microfluidics Tools: Single-Cell Approach
RBCs Deformability in Microfluidic Devices
| Microfluidic Device | Cell Types | Main Flow Phenomenon | Approach to Measure the Degree of Deformability | Configuration | Main Key Observations | References |
|---|---|---|---|---|---|---|
| Hyperbolic converging microchannels | Human RBCs (healthy and diseased) | Extensional flow | Deformation Ratio (DR)= |  | The proposed device is able to detect changes in DR of the RBCs, allowing for distinguishing the samples from the healthy controls and the patients. | Faustino et al., 2019 [50] |
| Hyperbolic converging microchannels | Human RBCs with magnetic NPs | Extensional flow | Deformation Index (DI)= |  | This microfluidic tool is capable of evaluating with high accuracy the impact of multifunctional nanoparticles designed for theranostic applications in contact with RBCs, using the proved extensional flow approach to measure with high accuracy the RBC’s DIs. | Rodrigues et al., 2016 [73] |
| Cross-flow microfluidic device with pillars | Human RBCs (healthy) | Cross-flow | Deformation Index (DI)= |  | The proposed microfluidic device has the potential to perform in one single step a partial passive separation of RBCs based on their deformability by measuring the optical absorption of the collected samples. | Faustino et al., 2018 [23] |
| Hyperbolic converging microchannels | Human RBCs (healthy and and artificially impaired) | Shear and extensional flow | Deformation Index (DI)= |  | This work is a valuable contribution to help establishing the development of new malaria diagnostic systems towards point-of-care devices. | Vilas Boas et al., 2018 [74] |
| Fire-shaped cylindrical glass micronozzles | Human RBCs (healthy and and chemically treated) | Extensional flow | Deformation Index (DI)= |  | The use of these types of micronozzles, whose fabrication is simple, fast, and uses low-cost equipment, to assess the deformability (DI) of microentities, have great potential to detect small changes of blood cell mechanical properties. | Rubio et al., 2019 [75] |
| Polydimethylsiloxane (PDMS) rectangular abrupt contractions | Human RBCs (healthy and chemically treated) | Shear flow | Deformation Index frequency of 10 kHz |  | The RBC with higher deformability is more stretched along the flow direction (a), resulting in larger gaps between the RBC membrane and channel walls than the less deformable RBC (b). RBC with higher deformability blocks less current than RBC with lower deformability. | Zeng et al., 2013 [78] |
3. Blood Analogues—Particulate Approaches
4. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baskurt, O.K.; Hardeman, M.R.; Rampling, M.W. Handbook of Hemorheology and Hemodynamics; IOS Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Baskurt, O.K.; Meiselman, H.J. Blood Rheology and Hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, J.J.; Popel, A.S.; Intaglietta, M.; Johnson, P.C. Rheological effects of red blood cell aggregation in the venous network: A review of recent studies. Biorheology 2001, 38, 263–274. [Google Scholar] [PubMed]
- De Cindio, B.; Gabriele, D.; Catapano, G.; Fata, P.; Hackel, R.; Bonofiglio, R. The blood rheology in renal pathology. Annali dell’Istituto Superiore di Sanita 2007, 43, 156–163. [Google Scholar] [PubMed]
- Tomaiuolo, G. Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics 2014, 8, 051501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Na, W.; Lee, S.B.; Ahn, C.W.; Moon, J.S.; Won, K.C.; Shin, S. Potential Diagnostic Hemorheological Indexes for Chronic Kidney Disease in Patients With Type 2 Diabetes. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Caprari, P.; Massimi, S.; Diana, L.; Sorrentino, F.; Maffei, L.; Materazzi, S.; Risoluti, R. Hemorheological Alterations and Oxidative Damage in Sickle Cell Anemia. Front. Mol. Biosci. 2019, 6, 142. [Google Scholar] [CrossRef]
- Wiewiora, M.; Piecuch, J.; Gluck, M.; Slowinska-Lozynska, L.; Sosada, K. The effects of weight loss surgery on blood rheology in severely obese patients. Surg. Obes. Relat. Dis. 2015, 11, 1307–1314. [Google Scholar] [CrossRef]
- Senen, K.; Topal, E.; Kilinc, E.; ten Cate, H.; Tek, I.; Karakoc, Y.; Yetkin, E. Plasma viscosity and mean platelet volume in patients undergoing coronary angiography. Clin. Hemorheol. Microcirc. 2010, 44, 35–41. [Google Scholar] [CrossRef]
- Cicco, G.; Pirrelli, A. Red blood cell (RBC) deformability, RBC aggregability and tissue oxygenation in hypertension. Clin. Hemorheol. Microcirc. 1999, 21, 169–177. [Google Scholar]
- Bessonov, N.; Sequeira, A.; Simakov, S.; Vassilevskii, Y.; Volpert, V. Methods of Blood Flow Modelling. Math. Model. Nat. Phenom. 2016, 11, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Babu, N.; Singh, M. Influence of hyperglycemia on aggregation, deformability and shape parameters of erythrocytes. Clin. Hemorheol. Microcirc. 2004, 31, 273–280. [Google Scholar] [PubMed]
- Peng, W.K.; Chen, L.; Boehm, B.O.; Han, J.; Loh, T.P. Molecular Phenotyping of Oxidative Stress in Diabetes Mellitus with Point-of-care NMR system. bioRxiv 2019, 565325. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.P.; Peng, W.K.; Chen, L.; Sethi, S.K. Application of smoothed continuous labile haemoglobin A1c reference intervals for identification of potentially spurious HbA1c results. J. Clin. Pathol. 2014, 67, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Connell, J.M.C. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef]
- Shin, S.; Hou, J.X.; Suh, J.S.; Singh, M. Validation and application of a microfluidic ektacytometer (RheoScan-D) in measuring erythrocyte deformability. Clin. Hemorheol. Microcirc. 2007, 37, 319–328. [Google Scholar]
- Yeom, E.; Lee, S.J. Microfluidic-based speckle analysis for sensitive measurement of erythrocyte aggregation: A comparison of four methods for detection of elevated erythrocyte aggregation in diabetic rat blood. Biomicrofluidics 2015, 9, 024110. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Zhao, Y. Rheological analysis of non-Newtonian blood flow using a microfluidic device. Sensors Actuators A Phys. 2011, 166, 207–213. [Google Scholar] [CrossRef]
- Brown, C.D.; Ghali, H.S.; Zhao, Z.; Thomas, L.L.; Friedman, E.L.I.A. Association of reduced red blood cell deformability and diabetic nephropathy. Kidney Int. 2005, 67, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Microfluidic approaches. Analyst 2019, 144, 87–113. [Google Scholar] [CrossRef]
- Lenshof, A.; Ahmad-Tajudin, A.; Järås, K.; Swärd-Nilsson, A.M.; Aberg, L.; Marko-Varga, G.; Malm, J.; Lilja, H.; Laurell, T. Acoustic whole blood plasmapheresis chip for prostate specific antigen microarray diagnostics. Anal. Chem. 2009, 81, 6030–6037. [Google Scholar] [CrossRef]
- Mellors, J.S.; Jorabchi, K.; Smith, L.M.; Ramsey, J.M. Integrated microfluidic device for automated single cell analysis using electrophoretic separation and electrospray ionization mass spectrometry. Anal. Chem. 2010, 82, 967–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faustino, V.; Catarino, S.O.; Pinho, D.; Lima, R.A.; Minas, G. A Passive Microfluidic Device Based on Crossflow Filtration for Cell Separation Measurements: A Spectrophotometric Characterization. Biosensors 2018, 8, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Huang, Y.; Wu, D.; Sun, J. Microfluidic-Based Single-Cell Study: Current Status and Future Perspective. Molecules 2018, 23, 2347. [Google Scholar] [CrossRef] [Green Version]
- Pinho, D.; Yaginuma, T.; Lima, R. A microfluidic device for partial cell separation and deformability assessment. BioChip J. 2013, 7, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.O.; Pinho, D.; Faustino, V.; Lima, R. A simple microfluidic device for the deformability assessment of blood cells in a continuous flow. Biomed. Microdevices 2015, 17, 108. [Google Scholar] [CrossRef]
- Siddhartha, T.; Kumar, Y.V.B.V.; Amit, P.; Suhas, S.J.; Amit, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromechan. Microeng. 2015, 25, 083001. [Google Scholar]
- Yu, Z.T.F.; Yong, K.M.A.; Fu, J. Microfluidic Blood Cell Preparation: Now and Beyond. Small 2014, 10, 1687–1703. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Cui, D.F.; Liu, C.C.; Li, H. Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sens. Actuators B Chem. 2008, 130, 216–221. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ishikawa, T.; Lima, R.; Matsuki, N.; Imai, Y.; Kaji, H.; Nishizawa, M.; Yamaguchi, T. Red blood cell motions in high-hematocrit blood flowing through a stenosed microchannel. J. Biomech. 2009, 42, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.K.; Paesani, D. Omics Meeting Onics: Towards the Next Generation of Spectroscopic-Based Technologies in Personalized Medicine. J. Pers. Med. 2019, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi Aria, M.; Erten, A.; Yalcin, O. Technology Advancements in Blood Coagulation Measurements for Point-of-Care Diagnostic Testing. Front. Bioeng. Biotechnol. 2019, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Gorgannezhad, L.; Stratton, H.; Nguyen, N.-T. Microfluidic-Based Nucleic Acid Amplification Systems in Microbiology. Micromachines 2019, 10, 408. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ju, R.; Sekine, S.; Zhang, D.; Zhuang, S.; Yamaguchi, Y. All-in-one microfluidic device for on-site diagnosis of pathogens based on an integrated continuous flow PCR and electrophoresis biochip. Lab Chip 2019, 19, 2663–2668. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Fritz, Z.; Novik, E.M.; Yarmush, G.M.; Schloss, R.S.; Zahn, J.D.; Yarmush, M.L. Microfluidic flow cytometry: The role of microfabrication methodologies, performance and functional specification. Technology 2018, 6, 1–23. [Google Scholar] [CrossRef]
- Ribeiro-Samy, S.; Oliveira, M.I.; Pereira-Veiga, T.; Muinelo-Romay, L.; Carvalho, S.; Gaspar, J.; Freitas, P.P.; López-López, R.; Costa, C.; Diéguez, L. Fast and efficient microfluidic cell filter for isolation of circulating tumor cells from unprocessed whole blood of colorectal cancer patients. Sci. Rep. 2019, 9, 8032. [Google Scholar] [CrossRef]
- Wang, X.; Yi, L.; Mukhitov, N.; Schrell, A.M.; Dhumpa, R.; Roper, M.G. Microfluidics-to-mass spectrometry: A review of coupling methods and applications. J. Chromatogr. A 2015, 1382, 98–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhagat, V. Microfluidics and Sensors for DNA Analysis. Int. J. Eng. Res. Sci. 2017, 3, 83–91. [Google Scholar]
- Zhang, Y.; Xiao, R.-R.; Yin, T.; Zou, W.; Tang, Y.; Ding, J.; Yang, J. Generation of Gradients on a Microfluidic Device: Toward a High-Throughput Investigation of Spermatozoa Chemotaxis. PLoS ONE 2015, 10, e0142555. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, J.; Yuan, D.; Li, W. Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 2016, 38, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.L.; Lu, H. Advances in microfluidic cell separation and manipulation. Curr. Opin. Chem. Eng. 2013, 2, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.; Huang, B.; Zhou, J.; Liang, Y.; Tian, J.; Ji, L.; Liang, X.; Ye, X. A Microfluidic Chip for Cell Patterning Utilizing Paired Microwells and Protein Patterns. Micromachines 2016, 8, 1. [Google Scholar] [CrossRef]
- Shinde, P.; Mohan, L.; Kumar, A.; Dey, K.; Maddi, A.; Patananan, N.A.; Tseng, F.-G.; Chang, H.-Y.; Nagai, M.; Santra, S.T. Current Trends of Microfluidic Single-Cell Technologies. Int. J. Mol. Sci. 2018, 19, 3143. [Google Scholar] [CrossRef] [Green Version]
- Toepfner, N.; Herold, C.; Otto, O.; Rosendahl, P.; Jacobi, A.; Kräter, M.; Stächele, J.; Menschner, L.; Herbig, M.; Ciuffreda, L.; et al. Detection of human disease conditions by single-cell morpho-rheological phenotyping of blood. eLife 2018, 7, e29213. [Google Scholar] [CrossRef]
- Faustino, V.; Rodrigues, R.O.; Pinho, D.; Costa, E.; Santos-Silva, A.; Miranda, V.; Amaral, J.S.; Lima, R. A Microfluidic Deformability Assessment of Pathological Red Blood Cells Flowing in a Hyperbolic Converging Microchannel. Micromachines 2019, 10, 645. [Google Scholar] [CrossRef] [Green Version]
- Bento, D.; Rodrigues, R.O.; Faustino, V.; Pinho, D.; Fernandes, C.S.; Pereira, A.I.; Garcia, V.; Miranda, J.M.; Lima, R. Deformation of red blood cells, air bubbles, and droplets in microfluidic devices: Flow visualizations and measurements. Micromachines 2018, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.O.; Sousa, P.; Gaspar, J.; Bañobre-López, M.; Lima, R.; Minas, G. Organ-on-a-chip: A Preclinical Microfluidic Platform for the Progress of Nanomedicine. Small 2020, 2003517, 1–19. [Google Scholar] [CrossRef]
- Peng, W.K.; Kong, T.F.; Ng, C.S.; Chen, L.; Huang, Y.; Bhagat, A.A.S.; Nguyen, N.-T.; Preiser, P.R.; Han, J. Micromagnetic resonance relaxometry for rapid label-free malaria diagnosis. Nat. Med. 2014, 20, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Dupré, A.; Lei, K.-M.; Mak, P.-I.; Martins, R.P.; Peng, W.K. Micro- and nanofabrication NMR technologies for point-of-care medical applications—A review. Microelectron. Eng. 2019, 209, 66–74. [Google Scholar] [CrossRef]
- Peng, W.K.; Chen, L.; Han, J. Development of miniaturized, portable magnetic resonance relaxometry system for point-of-care medical diagnosis. Rev. Sci. Instrum. 2012, 83, 095115. [Google Scholar] [CrossRef] [Green Version]
- Toh, R.J.; Peng, W.K.; Han, J.; Pumera, M. Direct In Vivo Electrochemical Detection of Haemoglobin in Red Blood Cells. Sci. Rep. 2014, 4, 6209. [Google Scholar] [CrossRef] [Green Version]
- Toh, R.J.; Peng, W.K.; Han, J.; Pumera, M. Haemoglobin electrochemical detection on various reduced graphene surfaces: Well-defined glassy carbon electrode outperforms the graphenoids. RSC Adv. 2014, 4, 8050–8054. [Google Scholar] [CrossRef]
- Tzeng, B.-B.; Sun, Y.-S. Design and Fabrication of a Microfluidic Viscometer Based on Electrofluidic Circuits. Micromachines 2018, 9, 375. [Google Scholar] [CrossRef] [Green Version]
- André, E.; Pannacci, N.; Dalmazzone, C.; Colin, A. A new way to measure viscosity in droplet-based microfluidics for high throughput analysis. Soft Matter 2019, 15, 504–514. [Google Scholar] [CrossRef]
- Magnusson, E.B.; Halldorsson, S.; Fleming, R.M.T.; Leosson, K. Real-time optical pH measurement in a standard microfluidic cell culture system. Biomed. Opt. Express 2013, 4, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Yamada, A.; Suzuki, M. A Microfluidic pH Measurement Device with a Flowing Liquid Junction. Sensors 2017, 17, 1563. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.-T.; Hejazian, M.; Ooi, H.C.; Kashaninejad, N. Recent Advances and Future Perspectives on Microfluidic Liquid Handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.; Kumar, Y.V.; Agrawal, A.; Prabhakar, A.; Joshi, S.S. Microdevice for plasma separation from whole human blood using bio-physical and geometrical effects. Sci. Rep. 2016, 6, 26749. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Nguyen, J.; Wei, Y.; Sun, Y. Recent advances in microfluidic techniques for single-cell biophysical characterization. Lab Chip 2013, 13, 2464–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Z.; Liu, H.; Zhang, Z.; Lin, C.; Wang, B. Hybrid magnetic and deformability based isolation of circulating tumor cells using microfluidics. AIP Adv. 2019, 9, 025023. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, T.; Nagamine, K.; Horiguchi, Y.; Shiku, H.; Koide, M.; Itayama, T.; Shiraishi, F.; Matsue, T. Electrophoretic Cell Manipulation and Electrochemical Gene-Function Analysis Based on a Yeast Two-Hybrid System in a Microfluidic Device. Anal. Chem. 2008, 80, 3722–3727. [Google Scholar] [CrossRef]
- Agrawal, R.; Smart, T.; Nobre-Cardoso, J.; Richards, C.; Bhatnagar, R.; Tufail, A.; Shima, D.; Jones, P.H.; Pavesio, C. Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci. Rep. 2016, 6, 15873. [Google Scholar] [CrossRef]
- Zeng, N.F.; Mancuso, J.E.; Zivkovic, A.M.; Smilowitz, J.T.; Ristenpart, W.D. Red Blood Cells from Individuals with Abdominal Obesity or Metabolic Abnormalities Exhibit Less Deformability upon Entering a Constriction. PLoS ONE 2016, 11, e0156070. [Google Scholar] [CrossRef] [Green Version]
- Barber, B.E.; Russell, B.; Grigg, M.J.; Zhang, R.; William, T.; Amir, A.; Lau, Y.L.; Chatfield, M.D.; Dondorp, A.M.; Anstey, N.M.; et al. Reduced red blood cell deformability in Plasmodium knowlesi malaria. Blood Adv. 2018, 2, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Rey, J.; Buffet, P.A.; Ciceron, L.; Milon, G.; Mercereau-Puijalon, O.; Safeukui, I. Reduced erythrocyte deformability associated with hypoargininemia during Plasmodiumfalciparum malaria. Sci. Rep. 2014, 4, 3767. [Google Scholar] [CrossRef] [Green Version]
- Kong, T.F.; Ye, W.; Peng, W.K.; Hou, H.W.; Preiser, P.R.; Nguyen, N.-T.; Han, J. Enhancing malaria diagnosis through microfluidic cell enrichment and magnetic resonance relaxometry detection. Sci. Rep. 2015, 5, 11425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, R.O.; Bañobre-López, M.; Gallo, J.; Tavares, P.B.; Silva, A.M.T.; Lima, R.; Gomes, H.T. Haemocompatibility of iron oxide nanoparticles synthesized for theranostic applications: A high-sensitivity microfluidic tool. J. Nanoparticle Res. 2016, 18, 194. [Google Scholar] [CrossRef] [Green Version]
- Boas, L.V.; Faustino, V.; Lima, R.; Miranda, J.M.; Minas, G.; Fernandes, C.S.V.; Catarino, S.O. Assessment of the Deformability and Velocity of Healthy and Artificially Impaired Red Blood Cells in Narrow Polydimethylsiloxane (PDMS) Microchannels. Micromachines 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio, A.; Faustino, V.; Cabezas, M.G.; Lima, R.; Vega, E.J. Fire-shaped cylindrical glass micronozzles to measure cell deformability. J. Micromechan. Microeng. 2019, 29, 105001. [Google Scholar] [CrossRef]
- Cruz, A.; Peng, W.K. Perspective: Cellular and Molecular Profiling Technologies in Personalized Oncology. J. Pers. Med. 2019, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Iliescu, F.S.; Poenar, D.P.; Yu, F.; Ni, M.; Chan, K.H.; Cima, I.; Taylor, H.K.; Cima, I.; Iliescu, C. Recent advances in microfluidic methods in cancer liquid biopsy. Biomicrofluidics 2019, 13, 041503. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Nguyen, J.; Wang, C.; Sun, Y. Electrical measurement of red blood cell deformability on a microfluidic device. Lab Chip 2013, 13, 3275–3283. [Google Scholar] [CrossRef]
- 7—Microfluidic Devices Based on Biomechanics. In Integrated Nano-Biomechanics; Yamaguchi, T.; Ishikawa, T.; Imai, Y. (Eds.) Elsevier: Boston, MA, USA, 2018; pp. 217–263. [Google Scholar] [CrossRef]
- Carvalho, V.; Maia, I.; Souza, A.; Ribeiro, J.; Costa, P.; Puga, H.; Teixeira, S.F.C.F.; Lima, R.A. In vitro stenotic arteries to perform blood analogues flow visualizations and measurements: A Review. Open Biomed. Eng. J. 2020, in press. [Google Scholar]
- Campo-Deaño, L.; Dullens, R.P.A.; Aarts, D.G.A.L.; Pinho, F.T.; Oliveira, M.S.N. Viscoelasticity of blood and viscoelastic blood analogues for use in polydymethylsiloxane in vitro models of the circulatory system. Biomicrofluidics 2013, 7, 034102. [Google Scholar] [CrossRef] [Green Version]
- Lieber, B.B.; Sadasivan, C.; Hao, Q.; Seong, J.; Cesar, L. The mixability of angiographic contrast with arterial blood. Med. Phys. 2009, 36, 5064–5078. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, V.; Rodrigues, N.; Ribeiro, R.; Costa, P.F.; Lima, R.A.; Teixeira, S.F.C.F. 3D Printed Biomodels for Flow Visualization in Stenotic Vessels: An Experimental and Numerical Study. Micromachines 2020, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.C.; Pinho, F.T.; Oliveira, M.S.N.; Alves, M.A. Extensional flow of blood analog solutions in microfluidic devices. Biomicrofluidics 2011, 5, 014108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broek, C.N. Medium with Blood-Analog Mechanical Properties for Cardiovascular Tissue Culturing; IOS Press: Eindhoven, The Netherlands, 2008; pp. 651–661. [Google Scholar]
- Fukada, E.; Seaman, G.V.F.; Liepsch, D.; Lee, M.; Friis-Baastad, L. Blood modeling using polystyrene microspheres. Biorheology 1989, 26, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Liepsch, D.; Thurston, G.; Lee, M. Studies of fluids simulating blood-like rheological properties and applications in models of arterial branches. Biorheology 1991, 28, 39–52. [Google Scholar] [CrossRef]
- Lerche, D.; Vlastos, G.; Koch, B.; Pohl, M.; Affeld, K. Viscoelastic behaviour of human blood and polyacrylamide model fluids for heart valve testing. J. Phys. 1993, 3, 1283–1289. [Google Scholar] [CrossRef]
- Vlastos, G.; Lerche, D.; Koch, B.; Samba, O.; Pohl, M. The effect of parallel combined steady and oscillatory shear flows on blood and polymer solutions. Rheol. Acta 1997, 36, 160–172. [Google Scholar] [CrossRef]
- Walker, A.M.; Johnston, C.R.; Rival, D.E. On the Characterization of a Non-Newtonian Blood Analog and Its Response to Pulsatile Flow Downstream of a Simplified Stenosis. Ann. Biomed. Eng. 2014, 42, 97–109. [Google Scholar] [CrossRef]
- Calejo, J.; Pinho, D.; Galindo-Rosales, F.; Lima, R.; Campo-Deaño, L. Particulate Blood Analogues Reproducing the Erythrocytes Cell-Free Layer in a Microfluidic Device Containing a Hyperbolic Contraction. Micromachines 2016, 7, 4. [Google Scholar] [CrossRef]
- Pinho, D.; Muñoz-Sánchez, B.N.; Vega, E.J.; Lima, R.; Pinho, F.T. Rheological behaviour of dextran suspensions of PDMS microbeads flowing through a hyperbolic microchannel. In Proceedings of the 7th Portuguese Congress on Biomecahnics, Guimarães, Portugal, 10–11 February 2017. [Google Scholar]
- Muñoz-Sánchez, B.N.; Silva, S.F.; Pinho, D.; Vega, E.J.; Lima, R. Generation of micro-sized PDMS particles by a flow focusing technique for biomicrofluidics applications. Biomicrofluidics 2016, 10, 014122. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Hyun, J.-C.; Yang, S. On-chip Extraction of Intracellular Molecules in White Blood Cells from Whole Blood. Sci. Rep. 2015, 5, 15167. [Google Scholar] [CrossRef]
- Pinho, D.; Muñoz-Sánchez, B.N.; Anes, C.F.; Vega, E.J.; Lima, R. Flexible PDMS microparticles to mimic RBCs in blood particulate analogue fluids. Mech. Res. Commun. 2019, 100, 103399. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Mongrain, R.; Prakash, S.; Tardif, J.C. Development of a Blood Analog for the Hernodynamic Efficiency Evaluation of Cardiovascular Devices. In Proceedings of the Canadian Design Engineering Network Conference, Montreal, QC, Canada, 29–30 July 2004. [Google Scholar]
- Chen, K.; Merkel, T.J.; Pandya, A.; Napier, M.E.; Luft, J.C.; Daniel, W.; Sheiko, S.; DeSimone, J.M. Low Modulus Biomimetic Microgel Particles with High Loading of Hemoglobin. Biomacromolecules 2012, 13, 2748–2759. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.A.M.; Rodrigues, A.R.O.; Faustino, V.; Pinho, D.; Castanheira, E.M.S.; Lima, R. Microfluidic Deformability Study of an Innovative Blood Analogue Fluid Based on Giant Unilamellar Vesicles. J. Funct. Biomater. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, R.; Vega, E.J.; Moita, A.S.; Miranda, J.M.; Pinho, D.; Moreira, A.L.N. Fast, flexible and low-cost multiphase blood analogue for biomedical and energy applications. Exp. Fluids 2020, 61, 231. [Google Scholar] [CrossRef]
- Carvalho, V.; Rodrigues, N.; Ribeiro, R.; Costa, P.F.; Teixeira, J.C.F.; Lima, R.A.; Teixeira, S.F.C.F. Hemodynamic study in 3D printed stenotic coronary artery models: Experimental validation and transient simulation. Comput. Methods Biomech. Biomed. Engin. 2020, 1–14. [Google Scholar] [CrossRef]
- Souza, A.; Souza, M.S.; Pinho, D.; Agujetas, R.; Ferrera, C.; Lima, R.; Puga, H.; Ribeiro, J. 3D manufacturing of intracranial aneurysm biomodels for flow visualizations: Low cost fabrication processes. Mech. Res. Commun. 2020, 107, 103535. [Google Scholar] [CrossRef]





| Analogue Fluid | Main Key Observations in Comparison to Whole Blood | References |
|---|---|---|
| Glycerol and water | Newtonian, macro behavior, viscosity curve | Lieber et al., 2009 [82] |
| Water and DMSO | Newtonian, macro behavior, viscosity curve | Carvalho et al., 2020 [83,100], Souza et al., 2020 [101] |
| Xanthan gum (XG) and PAA XG, PAA and glycerin | Non-Newtonian, macro behavior, hematocrit level, shear-thinning | Sousa et al., 2011 [84] |
| Dextran 40 and CaCl2 | Non-Newtonian, cell-free layer, micro behavior, hemodynamic phenomena, shear-thinning | Fukada et al., 1989 [86] |
| PAA | Non-Newtonian, shear-thinning behavior | Lerche et al., 1993 [88] |
| PAA and XG | Non-Newtonian, shear-thinning behavior, viscoelasticity | Vlastos et al., 1997 [89] |
| Sucrose, PAA and HA | Non-Newtonian, macro behavior, shear-thinning behavior, viscoelasticity | Campo-Deaño et al., 2013 [81] |
| Sucrose and xanthan gum | Non-Newtonian, macro behavior, shear-thinning behavior, viscoelasticity | Campo-Deaño et al., 2013 [81] |
| PMMA | Newtonian, micro behavior, pathological RBCs deformability | Calejo et al., 2016 [91] |
| PMMA particles, xanthan gum, Dx 40 | Non-Newtonian, micro behavior, shear-thinning behavior, viscoelasticity, pathological RBCs deformability, cell-free layer | Pinho et al., 2017 [92] |
| PDMS beads in Dx 40 | Non-Newtonian, micro behavior, shear-tinning behavior, RBCs deformability | Pinho et al., 2019 [95] |
| Hydrogel | Non-Newtonian, viscoelasticity | Nguyen et al., 2004 [96] |
| Microgel particles loaded with bovine Hb | Newtonian, micro behavior, RBCs deformability | Chen et al., 2012 [97] |
| Giant unilamellar vesicles, water | Newtonian, micro behavior, RBCs deformability | Carvalho et al., 2018 [98] |
| Brij L4 surfactant, water | Non-Newtonian, micro behavior, two-phase, shear-thinning behavior, cell-free layer, RBCs deformability | Lima et al., 2020 [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, D.; Carvalho, V.; Gonçalves, I.M.; Teixeira, S.; Lima, R. Visualization and Measurements of Blood Cells Flowing in Microfluidic Systems and Blood Rheology: A Personalized Medicine Perspective. J. Pers. Med. 2020, 10, 249. https://doi.org/10.3390/jpm10040249
Pinho D, Carvalho V, Gonçalves IM, Teixeira S, Lima R. Visualization and Measurements of Blood Cells Flowing in Microfluidic Systems and Blood Rheology: A Personalized Medicine Perspective. Journal of Personalized Medicine. 2020; 10(4):249. https://doi.org/10.3390/jpm10040249
Chicago/Turabian StylePinho, Diana, Violeta Carvalho, Inês M. Gonçalves, Senhorinha Teixeira, and Rui Lima. 2020. "Visualization and Measurements of Blood Cells Flowing in Microfluidic Systems and Blood Rheology: A Personalized Medicine Perspective" Journal of Personalized Medicine 10, no. 4: 249. https://doi.org/10.3390/jpm10040249