Evidence of Stable Foraminifera Biomineralization during the Last Two Climate Cycles in the Tropical Atlantic Ocean
Abstract
:1. Introduction
2. Location and Oceanographic Setting
3. Materials and Methods
3.1. Weight Analysis
3.2. Dissolution Assesment
3.3. X-ray Micro-Computed Tomography (μCT)
3.4. Geochemical Analysis
3.4.1. Oxygen Stable Isotope Determination
3.4.2. Mg/Ca Determination
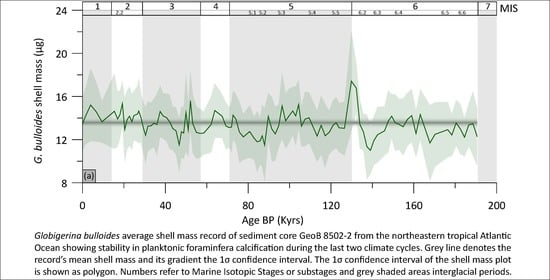
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Supporting Data
References
- Weinkauf, M.F.G.; Moller, T.; Koch, M.C.; Kučera, M. Calcification intensity in planktonic Foraminifera reflects ambient conditions irrespective of environmental stress. Biogeosciences 2013, 10, 6639–6655. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, G.P. A model for variation in the chemistry of planktonic foraminifera due to secondary calcification and selective dissolution. Paleoceanography 1995, 10, 445–457. [Google Scholar] [CrossRef]
- Barker, S.; Elderfield, H. Foraminiferal calcification response to glacial-interglacial changes in atmospheric CO2. Science 2002, 297, 833–836. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, S. Optimum growth conditions as opposed to calcite saturation as a control on the calcification rate and shell-weight of marine foraminifera. Mar. Biol. 2004, 144, 45–49. [Google Scholar] [CrossRef]
- Aldridge, D.; Beer, C.J.; Purdie, D.A. Calcification in the planktonic foraminifera Globigerina bulloides linked to phosphate concentrations in surface waters of the North Atlantic Ocean. Biogeosciences 2012, 9, 1725–1739. [Google Scholar] [CrossRef] [Green Version]
- Qin, B.; Li, T.; Xiong, Z.; Algeo, T.J.; Jia, Q. Calcification of planktonic foraminifer Pulleniatina obliquiloculata controlled by seawater temperature rather than ocean acidification. Glob. Planet. Chang. 2020, 193, 103256. [Google Scholar] [CrossRef]
- Zarkogiannis, S.D.; Antonarakou, A.; Tripati, A.; Kontakiotis, G.; Mortyn, P.G.; Drinia, H.; Greaves, M. Influence of surface ocean density on planktonic foraminifera calcification. Sci. Rep. 2019, 9, 533. [Google Scholar] [CrossRef]
- Zarkogiannis, S.D.; Kontakiotis, G.; Antonarakou, A.; Mortyn, P.G.; Drinia, H. Latitudinal Variation of Planktonic Foraminifera Shell Masses During Termination I. IOP Conf. Ser. Earth Environ. Sci. 2019, 221, 12052. [Google Scholar] [CrossRef]
- Marszalek, D.S. The role of heavy skeletons in vertical movements of non-motile zooplankton. Mar. Behav. Physiol. 1982, 8, 295–303. [Google Scholar] [CrossRef]
- Lipps, J.H. Ecology and paleoecology of planktic foraminifera. In Foraminiferal Ecology and Paleoecology; Lipps, J.H., Berger, W.H., Buzas, M.A., Douglas, R.G., Ross, C.A., Eds.; SEPM Society for Sedimentary Geology: Broken Arrow, OK, USA, 1979; Volume 6, pp. 62–104. [Google Scholar]
- Davis, C.V.; Badger, M.P.S.; Bown, P.R.; Schmidt, D.N. The response of calcifying plankton to climate change in the Pliocene. Biogeosciences 2013, 10, 6131–6139. [Google Scholar] [CrossRef] [Green Version]
- Barron, E.J. Tropical climate stability and implications for the distribution of life. In Effects of Past Global Change on Life; National Research Council, Ed.; The National Academies Press: Washington, DC, USA, 1995; pp. 108–117. [Google Scholar] [CrossRef]
- Newell, R.E.; Navato, A.R.; Hsiung, J. Long-term global sea surface temperature fluctuations and their possible influence on atmospheric CO2 concentrations. Pure Appl. Geophys. 1978, 116, 351–371. [Google Scholar] [CrossRef]
- Fox, L.; Stukins, S.; Hill, T.; Miller, C.G. Quantifying the Effect of Anthropogenic Climate Change on Calcifying Plankton. Sci. Rep. 2020, 10, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skonieczny, C.; Paillou, P.; Bory, A.; Bayon, G.; Biscara, L.; Crosta, X.; Eynaud, F.; Malaizé, B.; Revel, M.; Aleman, N.; et al. African humid periods triggered the reactivation of a large river system in Western Sahara. Nat. Commun. 2015, 6, 8751. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, S. A revised picture of the structure of the ‘monsoon’ and land ITCZ over West Africa. Clim. Dyn. 2009, 32, 1155–1171. [Google Scholar] [CrossRef]
- Tulet, P.; Mallet, M.; Pont, V.; Pelon, J.; Boone, A. The 7–13 March 2006 dust storm over West Africa: Generation, transport, and vertical stratification. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Holz, C.; Stuut, J.-B.W.; Henrich, R.; Meggers, H. Variability in terrigenous sedimentation processes off northwest Africa and its relation to climate changes: Inferences from grain-size distributions of a Holocene marine sediment record. Sediment. Geol. 2007, 202, 499–508. [Google Scholar] [CrossRef]
- Peyrillé, P.; Lafore, J.-P.; Redelsperger, J.-L. An Idealized Two-Dimensional Framework to Study the West African Monsoon. Part I: Validation and Key Controlling Factors. J. Atmos. Sci. 2007, 64, 2765–2782. [Google Scholar] [CrossRef]
- Aouni, A.E.; Daoudi, K.; Yahia, H.; Minaoui, K.; Benazzouz, A. Surface mixing and biological activity in the North-West African upwelling. Chaos Interdiscip. J. Nonlinear Sci. 2019, 29, 011104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittelstaedt, E. The ocean boundary along the northwest African coast: Circulation and oceanographic properties at the sea surface. Prog. Oceanogr. 1991, 26, 307–355. [Google Scholar] [CrossRef]
- Stramma, L.; Hόttl, S.; Schafstall, J. Water masses and currents in the upper tropical northeast Atlantic off northwest Africa. J. Geophys. Res. 2005, 110, C12006. [Google Scholar] [CrossRef] [Green Version]
- Tomczak, M. An analysis of mixing in the frontal zone of South and North Atlantic Central Water off North-West Africa. Prog. Oceanogr. 1981, 10, 173–192. [Google Scholar] [CrossRef]
- Tomczak, M.; Godfrey, J.S. Regional Oceanography: Introduction; Elsevier: New York, NY, USA, 1994; p. 422. [Google Scholar]
- Howe, J.N.W.; Piotrowski, A.M.; Noble, T.L.; Mulitza, S.; Chiessi, C.M.; Bayon, G. North Atlantic Deep Water Production during the Last Glacial Maximum. Nat. Commun. 2016, 7, 11765. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.; Kiefer, T.; Elderfield, H. Temporal changes in North Atlantic circulation constrained by planktonic foraminiferal shell weights. Paleoceanography 2004, 19, PA3008. [Google Scholar] [CrossRef]
- Beer, C.J.; Schiebel, R.; Wilson, P.A. Technical Note: On methodologies for determining the size-normalised weight of planktic foraminifera. Biogeosciences 2010, 7, 2193–2198. [Google Scholar] [CrossRef] [Green Version]
- Broecker, W.; Clark, E. An evaluation of Lohmann’s Foraminifera weight dissolution index. Paleoceanography 2001, 16, 531–534. [Google Scholar] [CrossRef]
- Thunell, R.C.; Honjo, S. Calcite dissolution and the modification of planktonic foraminiferal assemblages. Mar. Micropaleontol. 1981, 6, 169–182. [Google Scholar] [CrossRef]
- Volbers, A.N.A.; Henrich, R. Present water mass calcium carbonate corrosiveness in the eastern South Atlantic inferred from ultrastructural breakdown of Globigerina bulloides in surface sediments. Mar. Geol. 2002, 186, 471–486. [Google Scholar] [CrossRef]
- Zarkogiannis, S.; Fernandez, V.; Greaves, M.; Mortyn, P.G.; Kontakiotis, G.; Antonarakou, A. X-ray tomographic data of planktonic foraminifera species Globigerina bulloides from the Eastern Tropical Atlantic across Termination II. Gigabyte 2020, 1. [Google Scholar] [CrossRef]
- Iwasaki, S.; Kimoto, K.; Sasaki, O.; Kano, H.; Uchida, H. Sensitivity of planktic foraminiferal test bulk density to ocean acidification. Sci. Rep. 2019, 9, 9803. [Google Scholar] [CrossRef]
- Mashiotta, T.A.; Lea, D.W.; Spero, H.J. Glacial–interglacial changes in Subantarctic sea surface temperature and δ18O-water using foraminiferal Mg. Earth Planet. Sci. Lett. 1999, 170, 417–432. [Google Scholar] [CrossRef]
- Kim, S.-T.; O’Neil, J.R. Equilibrium and non-equilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Hut, G. Consultants group meeting on stable isotope reference samples for geochemical and hydrological investigations. In Proceedings of the Report to the Director General of the International Atomic Energy Agency, Vienna, Austria, 16–18 September 1985; p. 42. [Google Scholar]
- LeGrande, A.N.; Schmidt, G.A. Global gridded data set of the oxygen isotopic composition in seawater. Geophys. Res. Lett. 2006, 33, L12604. [Google Scholar] [CrossRef] [Green Version]
- Fairbanks, R.G. A 17,000-year glacio-eustatic sea level record: Influence of glacial melting rates on the Younger Dryas event and deep-ocean circulation. Nature 1989, 342, 637–642. [Google Scholar] [CrossRef]
- UNESCO. The International Thermodynamic Equation of Seawater—2010: Calculation and Use of Thermodynamic Properties; UNESCO: Paris, France, 2010; p. 196. [Google Scholar]
- Barker, S.; Greaves, M.; Elderfield, H. A study of cleaning procedures used for foraminiferal Mg/Ca paleothermometry. Geochem. Geophys. Geosyst. 2003, 4. [Google Scholar] [CrossRef]
- De Villiers, S.; Greaves, M.; Elderfield, H. An intensity ratio calibration method for the accurate determination of Mg/Ca and Sr/Ca of marine carbonates by ICP-AES. Geochem. Geophys. Geosyst. 2002, 3. [Google Scholar] [CrossRef]
- Greaves, M.; Barker, S.; Daunt, C.; Elderfield, H. Accuracy, standardization, and interlaboratory calibration standards for foraminiferal Mg/Ca thermometry. Geochem. Geophys. Geosyst. 2005, 6. [Google Scholar] [CrossRef]
- Greaves, M.; Caillon, N.; Rebaubier, H.; Bartoli, G.; Bohaty, S.; Cacho, I.; Clarke, L.; Cooper, M.; Daunt, C.; Delaney, M.; et al. Interlaboratory comparison study of calibration standards for foraminiferal Mg/Ca thermometry. Geochem. Geophys. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Rosenthal, Y.; Perron-Cashman, S.; Lear, C.H.; Bard, E.; Barker, S.; Billups, K.; Bryan, M.; Delaney, M.L.; de Menocal, P.B.; Dwyer, G.S.; et al. Interlaboratory comparison study of Mg/Ca and Sr/Ca measurements in planktonic foraminifera for paleoceanographic research. Geochem. Geophys. Geosyst. 2004, 5. [Google Scholar] [CrossRef]
- Petit, J.R.; Jouzel, J.; Raynaud, D.; Barkov, N.I.; Barnola, J.M.; Basile, I.; Bender, M.; Chappellaz, J.; Davis, M.; Delaygue, G.; et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 1999, 399, 429–436. [Google Scholar]
- Colleoni, F.; Wekerle, C.; Näslund, J.-O.; Brandefelt, J.; Masina, S. Constraint on the penultimate glacial maximum Northern Hemisphere ice topography (≈140 kyrs BP). Quat. Sci. Rev. 2016, 137, 97–112. [Google Scholar] [CrossRef]
- Siddall, M.; Rohling, E.J.; Almogi-Labin, A.; Hemleben, C.; Meischner, D.; Schmelzer, I.; Smeed, D.A. Sea-level fluctuations during the last glacial cycle. Nature 2003, 423, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wien, K.; Holz, C.; Kölling, M.; Schulz, H.D. Geochemical data (solid phase) of sediment core GeoB8502-2. Pangaea 2006. [Google Scholar] [CrossRef]
- Davis, C.V.; Rivest, E.B.; Hill, T.M.; Gaylord, B.; Russell, A.D.; Sanford, E. Ocean acidification compromises a planktic calcifier with implications for global carbon cycling. Sci. Rep. 2017, 7, 2225. [Google Scholar] [CrossRef]
- Liou, K.-N. Radiation climatology. In International Geophysics; Academic Press: Cambridge, MA, USA, 1980; Volume 26, pp. 293–348. [Google Scholar]
- Barker, S.; Archer, D.; Booth, L.; Elderfield, H.; Henderiks, J.; Rickaby, R.E.M. Globally increased pelagic carbonate production during the Mid-Brunhes dissolution interval and the CO2 paradox of MIS 11. Quat. Sci. Rev. 2006, 25, 3278–3293. [Google Scholar] [CrossRef]
- Johnson, G.C.; Schmidtko, S.; Lyman, J.M. Relative contributions of temperature and salinity to seasonal mixed layer density changes and horizontal density gradients. J. Geophys. Res. Oceans 2012, 117. [Google Scholar] [CrossRef]
- Moy, A.D.; Howard, W.R.; Bray, S.G.; Trull, T.W. Reduced calcification in modern Southern Ocean planktonic foraminifera. Nat. Geosci. 2009, 2, 276–280. [Google Scholar] [CrossRef]
- Berger, A. Milankovitch Theory and climate. Rev. Geophys. 1988, 26, 624–657. [Google Scholar] [CrossRef] [Green Version]
- Zarkogiannis, S.; Fernandez, V.; Greaves, M.; Mortyn, P.G.; Kontakiotis, G.; Antonarakou, A. Supporting data for “X-ray tomographic data of planktonic foraminifera species Globigerina bulloides from the Eastern Tropical Atlantic across Termination II”. GigaScience Database 2020. [Google Scholar] [CrossRef]
- Berger, W.H.; Bonneau, M.C.; Parker, F.L. Foraminifera on the deep-sea floor: Lysocline and dissolution rate. Oceanol. Acta 1982, 5, 249–258. [Google Scholar]
- Ruddiman, W.F. Pleistocene Sedimentation in the Equatorial Atlantic: Stratigraphy and Faunal Paleoclimatology. Geol. Soc. Am. Bull. 1971, 82, 283–302. [Google Scholar] [CrossRef]
- Curry, W.B.; Lohmann, G.P. Late Quaternary carbonate sedimentation at the Sierra Leone Rise (eastern equatorial Atlantic Ocean). Mar. Geol. 1986, 70, 223–250. [Google Scholar] [CrossRef]
- Prell, W.L. Late Pleistocene Faunal, Sedimentary and Temperature History of the Columbia Basin, Caribbean Sea; Columbia University: New York, NY, USA, 1974. [Google Scholar]
- Damuth, J.E. The Western Equatorial Atlantic Morphology, Quaternary Sediments, and Climatic Cycles; Columbia University: New York, NY, USA, 1973. [Google Scholar]
- Broecker, W.S.; Turekian, K.K.; Heezen, B.C. The relation of deep sea [Atlantic Ocean] sedimentation rates to variations in climate. Am. J. Sci. 1958, 256, 503–517. [Google Scholar] [CrossRef]
- Hays, J.D.; Perruzza, A. The Significance of Calcium Carbonate Oscillations in Eastern Equatorial Atlantic deep-Sea Sediments for the End of the Holocene Warm Interval. Quat. Res. 1972, 2, 355–362. [Google Scholar] [CrossRef]
- Bacon, M.P. Glacial to interglacial changes in carbonate and clay sedimentation in the Atlantic Ocean estimated from 230Th measurements. Chem. Geol. 1984, 46, 97–111. [Google Scholar] [CrossRef]
- Curry, W.B.; Cullen, J.L. Carbonate production and dissolution in the western equatorial Atlantic during the last 1 MY. In Proceedings of the Ocean Drilling Program, Scientific Results, Charleston, SC, USA, 8 January–14 February 1997; Volume 154, p. 189. [Google Scholar] [CrossRef]
- Eglinton, G.; Bradshaw, S.; Resell, A.; Sarnthein, M.; Pflaumann, U.; Tiedemann, R. Molecular record of secular sea surface temperature changes on 100-year timescales for glacial terminations I, II and IV. Nature 1992, 356, 423–426. [Google Scholar] [CrossRef]
- Speth, P.; Detlefsen, H.; Sierts, H.-W. Meteorological influence on upwelling off Northwest Africa. Ocean Dyn. 1978, 31, 95–104. [Google Scholar] [CrossRef]
- CLIMAP-Project-Members. The last interglacial ocean. Quat. Res. 1984, 21, 123–224. [Google Scholar] [CrossRef]
- Sarnthein, M.; Tiedemann, R. Younger Dryas-style cooling events at glacial Terminations I-VI at ODP Site 658: Associated benthic δ13C anomalies constrain meltwater hypothesis. Paleoceanography 1990, 5, 1041–1055. [Google Scholar] [CrossRef]
- Grant, K.M.; Rohling, E.J.; Ramsey, C.B.; Cheng, H.; Edwards, R.L.; Florindo, F.; Heslop, D.; Marra, F.; Roberts, A.P.; Tamisiea, M.E.; et al. Sea-level variability over five glacial cycles. Nat. Commun. 2014, 5, 5076. [Google Scholar] [CrossRef]
- Grant, K.M.; Rohling, E.J.; Bar-Matthews, M.; Ayalon, A.; Medina-Elizalde, M.; Ramsey, C.B.; Satow, C.; Roberts, A.P. Rapid coupling between ice volume and polar temperature over the past 150,000 years. Nature 2012, 491, 744–747. [Google Scholar] [CrossRef]
- Marino, G.; Rohling, E.J.; Rodríguez-Sanz, L.; Grant, K.M.; Heslop, D.; Roberts, A.P.; Stanford, J.D.; Yu, J. Bipolar seesaw control on last interglacial sea level. Nature 2015, 522, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Dupont, L.M.; Weinelt, M. Vegetation history of the savanna corridor between the Guinean and the Congolian rain forest during the last 150,000 years. Vegetat. Hist. Archaeobot. 1996, 5, 273–292. [Google Scholar] [CrossRef]
- Weldeab, S.; Lea, D.W.; Schneider, R.R.; Andersen, N. 155,000 years of West African monsoon and ocean thermal evolution. Science 2007, 316, 1303–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierro, F.J.; Hodell, D.A.; Andersen, N.; Azibeiro, L.A.; Jimenez-Espejo, F.J.; Bahr, A.; Flores, J.A.; Ausin, B.; Rogerson, M.; Lozano-Luz, R.; et al. Mediterranean Overflow over the Last 250 kyr: Freshwater Forcing from the Tropics to the Ice Sheets. Paleoceanogr. Paleoclimatol. 2020, 35, e2020PA003931. [Google Scholar] [CrossRef]
- Grant, K.M.; Grimm, R.; Mikolajewicz, U.; Marino, G.; Ziegler, M.; Rohling, E.J. The timing of Mediterranean sapropel deposition relative to insolation, sea-level and African monsoon changes. Quat. Sci. Rev. 2016, 140, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Coffey, M.; Dehairs, F.; Collette, O.; Luther, G.; Church, T.; Jickells, T. The Behaviour of Dissolved Barium in Estuaries. Estuar. Coast. Shelf Sci. 1997, 45, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Abouelmagd, A.; Sultan, M.; Milewski, A.; Kehew, A.E.; Sturchio, N.C.; Soliman, F.; Krishnamurthy, R.V.; Cutrim, E. Toward a better understanding of palaeoclimatic regimes that recharged the fossil aquifers in North Africa: Inferences from stable isotope and remote sensing data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 329–330, 137–149. [Google Scholar] [CrossRef]
- Kontakiotis, G.; Besiou, E.; Antonarakou, A.; Zarkogiannis, S.D.; Kostis, A.; Mortyn, P.G.; Moissette, P.; Cornée, J.J.; Schulbert, C.; Drinia, H.; et al. Decoding sea surface and paleoclimate conditions in the eastern Mediterranean over the Tortonian-Messinian Transition. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 534, 109312. [Google Scholar] [CrossRef]





| Sample | Age (kyrs) | Test Mass (μg) | Test Thickness (μm) | Cell Volume (μm3) | Sediment Infilling | Shell Density (g/cm3) | Test Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| 785 | 122.5 | 13.6 ± 2.4 | 5.0 ± 0.4 | 22,326,089 ± 11% | 5% | 0.61 ± 0.12 | 35 ± 7 |
| 825 | 132.2 | 16.8 ± 2.2 | 5.7 ± 0.5 | 24,126,844 ± 22% | 19% | 0.69 ± 0.18 | 21 ± 6 |
| 865 | 139.1 | 11.0 ± 2.2 | 4.8 ± 0.5 | 19,091,400 ± 17% | 4% | 0.58 ± 0.15 | 17 ± 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarkogiannis, S.D.; Antonarakou, A.; Fernandez, V.; Mortyn, P.G.; Kontakiotis, G.; Drinia, H.; Greaves, M. Evidence of Stable Foraminifera Biomineralization during the Last Two Climate Cycles in the Tropical Atlantic Ocean. J. Mar. Sci. Eng. 2020, 8, 737. https://doi.org/10.3390/jmse8100737
Zarkogiannis SD, Antonarakou A, Fernandez V, Mortyn PG, Kontakiotis G, Drinia H, Greaves M. Evidence of Stable Foraminifera Biomineralization during the Last Two Climate Cycles in the Tropical Atlantic Ocean. Journal of Marine Science and Engineering. 2020; 8(10):737. https://doi.org/10.3390/jmse8100737
Chicago/Turabian StyleZarkogiannis, Stergios D., Assimina Antonarakou, Vincent Fernandez, P. Graham Mortyn, George Kontakiotis, Hara Drinia, and Mervyn Greaves. 2020. "Evidence of Stable Foraminifera Biomineralization during the Last Two Climate Cycles in the Tropical Atlantic Ocean" Journal of Marine Science and Engineering 8, no. 10: 737. https://doi.org/10.3390/jmse8100737