Hyperspectral Imaging Using Laser Excitation for Fast Raman and Fluorescence Hyperspectral Imaging for Sorting and Quality Control Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Design and Software
2.2. Spectral Calibration and Spectral Resolution
2.3. Spatial Resolution
2.4. Example Measurements
2.5. Classification Experiments
3. Results
3.1. System Design
3.2. Spectral Calibration and Spectral and Spatial Resolution
3.3. Example Measurements
3.3.1. Spatial Distribution of Naphthalene Granules in a Sulphur Matrix (a)
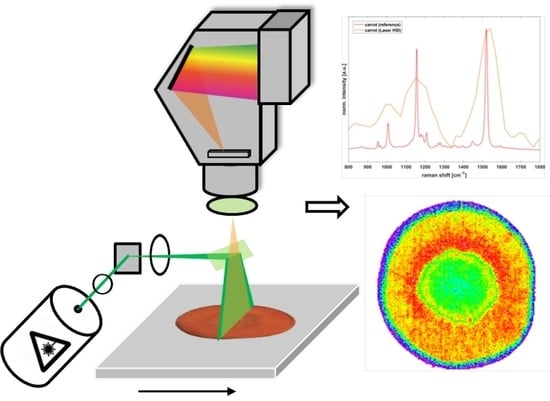
3.3.2. Carotenoid Distribution in a Carrot Slice (b)
3.3.3. Black Polymer Sorting (c)
3.3.4. Contaminations on PZT Piezoelectric Actuators (d)
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borengasser, M.; Hungate, W.S. Hyperspectral Remote Sensing. Principles and Applications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Dale, L.M.; Thewis, A.; Boudry, C.; Rotar, I.; Dardenne, P.; Baeten, V.; Pierna, J.A.F. Hyperspectral imaging applications in agriculture and agro-food product quality and safety control: A review. Appl. Spectrosc. Rev. 2013, 48, 142–159. [Google Scholar] [CrossRef]
- Elmasry, G.; Kamruzzaman, M.; Sun, D.W.; Allen, P. Principles and applications of hyperspectral imaging in quality evaluation of agro-food products: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 999–1023. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.A.; O’Donnell, C.P.; Cullen, P.J.; Bell, S.E.J. Recent applications of chemical imaging to pharmaceutical process monitoring and quality control. Eur. J. Pharm. Biopharma. 2008, 69, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gendrin, C.; Roggo, Y.; Collet, C. Pharmaceutical applications of vibrational chemical imaging and chemometrics: A review. J. Pharm. Biomed. Anal. 2008, 48, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.A.; Parasca, S.V.; Savastru, D.; Manea, D. Hyperspectral imaging in the medical field: present and future. Appl. Spectrosc. Rev. 2013, 49, 435–447. [Google Scholar] [CrossRef]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 10901. [Google Scholar] [CrossRef] [PubMed]
- Tilling, A.K.; O’Leary, G.J.; Ferwerda, J.G.; Jones, S.D.; Fitzgerald, G.J.; Rodriguez, D.; Belford, R. Remote sensing of nitrogen and water stress in wheat. Field Crop. Res. 2007, 104, 77–85. [Google Scholar] [CrossRef]
- Lewis, E.N.; Kidder, L.H.; Lee, E. NIR chemical imaging—Near infrared spectroscopy on steroids. NIR News 2005, 16, 2–4. [Google Scholar] [CrossRef]
- Martin, M.E.; Wabuyele, M.B.; Chen, K.; Kasili, P.; Panjehpour, M.; Phan, M.; Overholt, B.; Cunningham, G.; Wilson, D.; DeNovo, R.C.; et al. Development of an Advanced Hyperspectral Imaging (HSI) System with Applications for Cancer Detection. Ann. Biomed. Eng. 2006, 34, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.; Odonell, C.; Cullen, P.; Downey, G.; Frias, J. Hyperspectral imaging—An emerging process analytical tool for food quality and safety control. Trends Food Sci. Tech. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Boldrini, B.; Kessler, W.; Rebner, K.; Kessler, R.W. Hyperspectral imaging: A review of best practice, performance and pitfalls for in-line and on-line applications. J. Near Infrared Spec. 2012, 20, 483–508. [Google Scholar] [CrossRef]
- Stewart, S.; Priore, R.J.; Nelson, M.P.; Treado, P.J. Raman imaging. Ann. Rev. Anal. Chem. 2012, 5, 337–360. [Google Scholar] [CrossRef] [PubMed]
- Févotte, G. In situ Raman spectroscopy for in-line control of pharmaceutical crystallization and solids elaboration processes: A review. Chem. Eng. Res. Des. 2007, 85, 906–920. [Google Scholar] [CrossRef]
- Adar, F.; Geiger, R.; Noonan, J. Raman spectroscopy for process/quality control. Appl. Spectrosc. Rev. 1997, 32, 45–101. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Zimmermann, T.; Rietdorf, J.; Pepperkok, R. Spectral imaging and its applications in live cell microscopy. FEBS Lett. 2003, 546, 87–92. [Google Scholar] [CrossRef] [Green Version]
- El-Mashtoly, S.F.; Petersen, D.; Yosef, H.K.; Mosig, A.; Reinacher-Schick, A.; Kötting, C.; Gerwert, K. Label-free imaging of drug distribution and metabolism in colon cancer cells by Raman microscopy. Analyst 2014, 139, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Hartschuh, A.; Sánchez, E.J.; Xie, X.S.; Novotny, L. High-resolution near-field Raman microscopy of single-walled carbon nanotubes. Phys. Rev. Lett. 2003, 90, 95503. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Raman Spectroscopy for Chemical Analysis; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Qin, J.; Chao, K.; Kim, M.S. High-throughput Raman chemical imaging for evaluating food safety and quality. In Proceedings of the Sensing for Agriculture and Food Quality and Safety VI, San Francisco, CA, USA, 1–6 February 2014; p. 91080F. [Google Scholar]
- Qin, J.; Kim, M.S.; Chao, K.; Schmidt, W.F.; Cho, B.K.; Delwiche, S.R. Line-scan Raman imaging and spectroscopy platform for surface and subsurface evaluation of food safety and quality. J. Food Eng. 2017, 198, 17–27. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Palonpon, A.F.; Ando, J.; Yamakoshi, H.; Dodo, K.; Sodeoka, M.; Kawata, S.; Fujita, K. Raman and SERS microscopy for molecular imaging of live cells. Nat. Protoc. 2013, 8, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Baranski, R.; Schulz, H.; Nothnagel, T. Tissue-specific accumulation of carotenoids in carrot roots. Planta 2006, 224, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Mazet, V.; Carteret, C.; Brie, D.; Idier, J.; Humbert, B. Background removal from spectra by designing and minimising a non-quadratic cost function. Chemometr. Intell. Lab. 2005, 76, 121–133. [Google Scholar] [CrossRef]
- Camargo, E.R.; Frantti, J.; Kakihana, M. Low-temperature chemical synthesis of lead zirconate titanate (PZT) powders free from halides and organics. J. Mater. Chem. 2001, 11, 1875–1879. [Google Scholar] [CrossRef]





| Experiment | Lens | Laser Power (mW) | Integration Time (ms) | Speed (mm·s−1) | Frame rate (Hz) | Binning (Lateral × Spectral) | Total Time (s) | Total Area (cm2) |
|---|---|---|---|---|---|---|---|---|
| (a) sulphur and naphthalene | Xenoplan | 150 | 25 | 2.4 | 24 | 2 × 1 | 20 | 50 |
| (b) carrot slice | Xenoplan | 300 | 100 | 0.8 | 8 | 2 × 1 | 50 | 42 |
| (c) black polymers | Xenoplan | 150 | 25 | 2.4 | 24 | 2 × 1 | 42 | 105 |
| (d) PZT actuator | Sill | 300 | 100 | 0.013 | 8 | 4 × 1 | 170 | 2.3 |
| Slit Width (μm) | Wavelength Interval (nm) | Mean Absolute Error (nm) | FWHM (583 nm) (nm) | FWHM (702 nm) (nm) | FWHM (583 nm) (cm−1) | FWHM (702 nm) (cm−1) |
|---|---|---|---|---|---|---|
| 25 | 0.79 | 0.25 | 1.8 | 2.0 | 52.9 | 40.6 |
| 40 | 0.8 | 0.58 | 2.8 | 3.1 | 82.3 | 63.0 |
| 60 | 0.79 | 1.28 | 4.1 | 4.5 | 120.5 | 91.4 |
| Lens | Working Distance (mm) | FOV (mm) | FWHM (pixel) | Spatial Resolution (mm) |
|---|---|---|---|---|
| Cinegon, f/1.4, 8 mm | 330 | 386 | 3.49 | 1.34 |
| Xenoplan, f/1.4, 28 mm | 330 | 104 | 3.97 | 0.41 |
| Sill, S5LPJ2426 | 85.7 | 4 | 4.18 | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, F.; Wollmann, P.; Grählert, W.; Kaskel, S. Hyperspectral Imaging Using Laser Excitation for Fast Raman and Fluorescence Hyperspectral Imaging for Sorting and Quality Control Applications. J. Imaging 2018, 4, 110. https://doi.org/10.3390/jimaging4100110
Gruber F, Wollmann P, Grählert W, Kaskel S. Hyperspectral Imaging Using Laser Excitation for Fast Raman and Fluorescence Hyperspectral Imaging for Sorting and Quality Control Applications. Journal of Imaging. 2018; 4(10):110. https://doi.org/10.3390/jimaging4100110
Chicago/Turabian StyleGruber, Florian, Philipp Wollmann, Wulf Grählert, and Stefan Kaskel. 2018. "Hyperspectral Imaging Using Laser Excitation for Fast Raman and Fluorescence Hyperspectral Imaging for Sorting and Quality Control Applications" Journal of Imaging 4, no. 10: 110. https://doi.org/10.3390/jimaging4100110