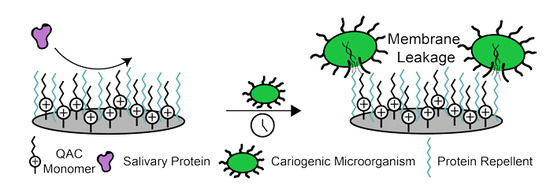
Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials
Abstract
:1. Introduction
2. Theoretical and Practical Considerations for Protein Adsorption
3. Characterization Methods for Quantifying Protein Adsorption
4. Dental Materials with Protein-Repellent Functionality
5. Mouthwash Coating Technology
| Protein Repellent Compound | Bulk Material | Filler | Adsorption Value (ng/cm2) | Quantification Method | Reference |
|---|---|---|---|---|---|
| 3% MPC (w/w) | 25.5% 1:1 BisGMA/TEGDMA | 70% Barium boroaluminosilicate | 1240 | SDS removal + BCA Assay | [58] |
| 3% MPC | 27% 1:1 BisGMA/TEGDMA | 70% Barium boroaluminosilicate | 960 | SDS removal + BCA Assay | [66] |
| 7.5% MPC | 75% 1:1 Scotchbond Multi-Purpose Primer and Adhesive | 15% Amorphous calcium phosphate | 321 | SDS removal + BCA Assay | [68] |
| 3% MPC | 25.5% 50:50 BisGMA/TEGDMA | 70% Barium boroaluminosilicate | 972 (with 180 days water aging) | SDS removal + BCA Assay | [69] |
| 3% MPC | 24% 1:1 EBPM | 20% Amorphous calcium phosphate; 50% barium boroaluminosilicate | 1200 | SDS removal + BCA Assay | [70] |
| 3% MPC | 44.5% PMGDM, 39.5% EBPADMA, 10% 2-hydroxyethyl methacrylate, 5% BisGMA | 30% Amorphous calcium phosphate | 416 | SDS removal + BCA Assay | [72] |
| 3% MPC | 47.75% Nature CrylTM liquid | 47.75% Nature CrylTM powder | 2150 | SDS removal + BCA Assay | [74] |
| 3% MPC | 24% 1:1 EBPM | 20% NACP, 50% barium boroaluminosilicate | 1000 | SDS removal + BCA Assay | [76] |
| PEG | Self-assembled PEG lysozyme | N/A | 8 | QCM | [53] |
| 33% trimethylamine N-oxide Zwitterionic Hydrogel | N/A | N/A | 3 * | SPR | [80] |
| 9% Poly(carboxybetaine acrylamide) Zwitterionic Hydrogel | N/A | N/A | 4.3 * | SPR | [49] |
6. Limitations of Existing Technologies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Sugars and Dental Caries. Available online: https://www.who.int/news-room/fact-sheets/detail/sugars-and-dental-caries (accessed on 15 June 2020).
- Venhoven, B.A.M.; de Gee, A.J.; Davidson, C.L. Polymerization contraction and conversion of light-curing BisGMA-based methacrylate resins. Biomaterials 1993, 14, 871–875. [Google Scholar] [CrossRef]
- O’Donnell, J.N.R.; Skrtic, D. Degree of Vinyl Conversion, Polymerization Shrinkage and Stress Development in Experimental Endodontic Composite. J. Biomim. Biomater. Tissue Eng. 2009, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, Y.; Yu, J.; Sun, Y.; Xie, W. Study of POSS on the Properties of Novel Inorganic Dental Composite Resin. Polymers 2020, 12, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wu, X.; Sun, Y.; Xie, W. POSS Dental Nanocomposite Resin: Synthesis, Shrinkage, Double Bond Conversion, Hardness, and Resistance Properties. Polymers 2018, 10, 369. [Google Scholar] [CrossRef] [Green Version]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J. Res. Natl. Inst. Stand. Technol. 2003, 108, 167. [Google Scholar] [CrossRef]
- Tanaka, J.; Inoue, K.; Masamura, H.; Matsumura, K.; Najai, H.; Inoue, K. The Application of Fluorinated Aromatic Dimethacrylates to Experimental Light-cured Radiopaque Composite Resin, Containing Barium-Borosilicate Glass Filler—A Progress in Nonwaterdegradable Properties. Dent. Mater. J. 1993, 12, 1–11. [Google Scholar] [CrossRef]
- Arcís, R.W.; López-Macipe, A.; Toledano, M.; Osorio, E.; Rodríguez-Clemente, R.; Murtra, J.; Fanovich, M.A.; Pascual, C.D. Mechanical properties of visible light-cured resins reinforced with hydroxyapatite for dental restoration. Dent. Mater. 2002, 18, 49–57. [Google Scholar] [CrossRef]
- Kuper, N.K.; van de Sande, F.H.; Opdam, N.J.M.; Bronkhorst, E.M.; de Soet, J.J.; Cenci, M.S.; Huysmans, M.C.D.J.N.M. Restoration Materials and Secondary Caries Using an In Vitro Biofilm Model. J. Dent. Res. 2015, 94, 62–68. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef]
- Imazato, S.; Torri, M.; Tsuchitani, Y. Immobilization of an antibacterial component in composite resin. Dent. Jpn. 1993, 30, 63–68. [Google Scholar]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R.B. Incorporation of Bacterial Inhibitor into Resin Composite. J. Dent. Res. 1994. [Google Scholar] [CrossRef]
- Gottenbos, B.; van der Mei, H.C.; Klatter, F.; Nieuwenhuis, P.; Busscher, H.J. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 2002, 23, 1417–1423. [Google Scholar] [CrossRef]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of Quaternary Ammonium Chain Length on Antibacterial Bonding Agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Gozzelino, G.; Lisanti, C.; Beneventi, S. Quaternary ammonium monomers for UV crosslinked antibacterial surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 21–28. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of Methacrylate Monomers with Antibacterial Effects Against S. Mutans. Molecules 2011, 16, 9755–9763. [Google Scholar] [CrossRef] [Green Version]
- Bienek, D.R.; Giuseppetti, A.A.; Okeke, U.C.; Frukhtbeyn, S.A.; Dupree, P.J.; Khajotia, S.S.; Florez, F.L.E.; Hiers, R.D.; Skrtic, D. Antimicrobial, biocompatibility, and physicochemical properties of novel adhesive methacrylate dental monomers. J. Bioact. Compat. Polym. 2020. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienek, D.; Frukhtbeyn, S.; Giuseppetti, A.; Okeke, U.; Skrtic, D. Antimicrobial Monomers for Polymeric Dental Restoratives: Cytotoxicity and Physicochemical Properties. JFB 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daood, U.; Parolia, A.; Elkezza, A.; Yiu, C.K.; Abbott, P.; Matinlinna, J.P.; Fawzy, A.S. An in vitro study of a novel quaternary ammonium silane endodontic irrigant. Dent. Mater. 2019, 35, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Bienek, D.R.; Giuseppetti, A.A.; Frukhtbeyn, S.A.; Hiers, R.D.; Esteban Florez, F.L.; Khajotia, S.S.; Skrtic, D. Physicochemical, Mechanical, and Antimicrobial Properties of Novel Dental Polymers Containing Quaternary Ammonium and Trimethoxysilyl Functionalities. JFB 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daood, U.; Matinlinna, J.P.; Pichika, M.R.; Mak, K.-K.; Nagendrababu, V.; Fawzy, A.S. A quaternary ammonium silane antimicrobial triggers bacterial membrane and biofilm destruction. Sci. Rep. 2020, 10, 10970. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Ten Cate, J.M. Biofilms, a new approach to the microbiology of dental plaque. Odontology 2006, 94, 1–9. [Google Scholar] [CrossRef]
- Saini, R.; Saini, S.; Sharma, S. Biofilm: A dental microbial infection. J. Nat. Sci. Biol. Med. 2011, 2, 71. [Google Scholar] [CrossRef] [Green Version]
- Hojo, K.; Nagaoka, S.; Ohshima, T.; Maeda, N. Bacterial Interactions in Dental Biofilm Development. J. Dent. Res. 2009, 88, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Hay, D.I. Adsorbed Salivary Acidic Proline-rich Proteins Contribute to the Adhesion of Streptococcus mutans JBP to Apatitic Surfaces. J. Dent. Res. 1989, 68, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Misra, A.; Goncalves, S.E.P.; Laurence, J.S. Proteins, Pathogens, and Failure at the Composite-Tooth Interface. J. Dent. Res. 2014, 93, 1243–1249. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofim in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94. [Google Scholar]
- Castagnola, M.; Scarano, E.; Passali, G.C.; Messana, I.; Cabras, T.; Iavarone, F.; Cintio, G.D.; Fiorita, A.; Corso, E.D.; Paludetti, G. Salivary biomarkers and proteomics: Future diagnostic and clinical utilities. Acta Otorhinolaryngolog. Italica 2017, 37, 94. [Google Scholar]
- McPherson, T.B.; Lee, S.J.; Park, K. Analysis of the Prevention of Protein Adsorption by Steric Repulsion Theory. In Proteins at Interfaces II; Horbett, T.A., Brash, J.L., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1995; Volume 602, pp. 395–404. ISBN 978-0-8412-3304-1. [Google Scholar]
- Müller, C.; Wald, J.; Hoth-Hannig, W.; Umanskaya, N.; Scholz, D.; Hannig, M.; Ziegler, C. Protein adhesion on dental surfaces—A combined surface analytical approach. Anal. Bioanal. Chem. 2011, 400, 679–689. [Google Scholar] [CrossRef]
- Tilton, R.D.; Robertson, C.R.; Gast, A.P. Manipulation of hydrophobic interactions in protein adsorption. Langmuir 1991, 7, 2710–2718. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- KL Prime; G Whitesides Self-assembled organic monolayers: Model systems for studying adsorption of proteins at surfaces. Science 1991, 252, 1164–1167. [CrossRef] [Green Version]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Chapman, R.G.; Ostuni, E.; Takayama, S.; Holmlin, R.E.; Yan, L.; Whitesides, G.M. Surveying for Surfaces that Resist the Adsorption of Proteins. J. Am. Chem. Soc. 2000, 122, 8303–8304. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Chen, X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMs that Resist Nonspecific Adsorption of Protein from Aqueous Buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef]
- Bernhard, C.; Roeters, S.J.; Franz, J.; Weidner, T.; Bonn, M.; Gonella, G. Repelling and ordering: The influence of poly(ethylene glycol) on protein adsorption. Phys. Chem. Chem. Phys. 2017, 19, 28182–28188. [Google Scholar] [CrossRef] [Green Version]
- Baggerman, J.; Smulders, M.M.J.; Zuilhof, H. Romantic Surfaces: A Systematic Overview of Stable, Biospecific, and Antifouling Zwitterionic Surfaces. Langmuir 2019, 35, 1072–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.; Hung, H.-C.; Lin, X.; Ma, J.; Zhang, P.; Sun, F.; Wu, K.; Jiang, S. Poly(ectoine) Hydrogels Resist Nonspecific Protein Adsorption. Langmuir 2017, 33, 11264–11269. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-N.; Sun, F.; Hung, H.-C.; Jain, P.; Sinclair, A.; Zhang, P.; Bai, T.; Chang, Y.; Wen, T.-C.; Yu, Q.; et al. Ultra-low fouling and high antibody loading zwitterionic hydrogel coatings for sensing and detection in complex media. Acta Biomater. 2016, 40, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabaté del Río, J.; Henry, O.Y.F.; Jolly, P.; Ingber, D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef]
- Migliorini, E.; Weidenhaupt, M.; Picart, C. Practical guide to characterize biomolecule adsorption on solid surfaces (Review). Biointerphases 2018, 13, 06D303. [Google Scholar] [CrossRef] [Green Version]
- Prabowo, B.; Purwidyantri, A.; Liu, K.-C. Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lu, D.; Deng, J.; Zhang, X.; Yang, P. Amyloid-Like Rapid Surface Modification for Antifouling and In-Depth Remineralization of Dentine Tubules to Treat Dental Hypersensitivity. Adv. Mater. 2019, 31, 1903973. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, S.A.; Evans, E.; Benavidez, T.E.; Garcia, C.D. Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: A review. Anal. Chim. Acta 2015, 872, 7–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höök, F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B Biointerfaces 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Ash, A.; Mulholland, F.; Burnett, G.R.; Wilde, P.J. Structural and compositional changes in the salivary pellicle induced upon exposure to SDS and STP. Biofouling 2014, 30, 1183–1197. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Coriand, L.; Loeffler, R.; Geis-Gerstorfer, J.; Zhou, Y.; Scheideler, L.; Fleischer, M.; Gehring, F.K.; Rupp, F. Saliva-coated titanium biosensor detects specific bacterial adhesion and bactericide caused mass loading upon cell death. Biosens. Bioelectron. 2019, 129, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, J.; Melo, M.A.S.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratz, F.; Grass, S.; Umanskaya, N.; Scheibe, C.; Müller-Renno, C.; Davoudi, N.; Hannig, M.; Ziegler, C. Cleaning of biomaterial surfaces: Protein removal by different solvents. Colloids Surf. B Biointerfaces 2015, 128, 28–35. [Google Scholar] [CrossRef]
- Kwon, J.-S.; Lee, M.-J.; Kim, J.-Y.; Kim, D.; Ryu, J.-H.; Jang, S.; Kim, K.-M.; Hwang, C.-J.; Choi, S.-H. Novel anti-biofouling light-curable fluoride varnish containing 2-methacryloyloxyethyl phosphorylcholine to prevent enamel demineralization. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-J.; Kwon, J.-S.; Kim, J.-Y.; Ryu, J.-H.; Seo, J.-Y.; Jang, S.; Kim, K.-M.; Hwang, C.-J.; Choi, S.-H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Davies, M.C.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M.; Davies, J.; Dawkes, A.C.; Edwards, J.C. Recognition of Protein Adsorption onto Polymer Surfaces by Scanning Force Microscopy and Probe−Surface Adhesion Measurements with Protein-Coated Probes. Langmuir 1997, 13, 4106–4111. [Google Scholar] [CrossRef]
- Cao, L.; Wu, J.; Zhang, Q.; Baras, B.; Bhadila, G.; Li, Y.; Melo, M.A.S.; Weir, M.D.; Bai, Y.; Zhang, N.; et al. Novel Protein-Repellent and Antibacterial Resins and Cements to Inhibit Lesions and Protect Teeth. Int. J. Polym. Sci. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Zhang, K.; Xie, X.; Dai, Z.; Zhao, Z.; Imazato, S.; Al-Dulaijan, Y.; Al-Qarni, F.; Weir, M.; Reynolds, M.; et al. Nanostructured Polymeric Materials with Protein-Repellent and Anti-Caries Properties for Dental Applications. Nanomaterials 2018, 8, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Chen, C.; Melo, M.A.; Bai, Y.-X.; Cheng, L.; Xu, H.H. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int. J. Oral Sci. 2015, 7, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Weir, M.D.; Romberg, E.; Bai, Y.; Xu, H.H.K. Development of novel dental adhesive with double benefits of protein-repellent and antibacterial capabilities. Dent. Mater. 2015, 31, 845–854. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Chen, C.; Liu, J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent. Mater. 2015, 31, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Zhang, K.; Melo, M.; Weir, M.; Xu, D.; Bai, Y.; Xu, H. Effects of Long-Term Water-Aging on Novel Anti-Biofilm and Protein-Repellent Dental Composite. IJMS 2017, 18, 186. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Imazato, S.; Weir, M.D.; Reynolds, M.A.; Xu, H.H.K. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater. Sci. Eng. C 2016, 67, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Weir, M.D.; Chow, L.C.; Antonucci, J.M.; Chen, J.; Xu, H.H.K. Novel rechargeable calcium phosphate dental nanocomposite. Dent. Mater. 2016, 32, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Al-Qarni, F.D.; Tay, F.; Weir, M.D.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Xie, X.; Xu, H.H.K. Protein-repelling adhesive resin containing calcium phosphate nanoparticles with repeated ion-recharge and re-releases. J. Dent. 2018, 78, 91–99. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Weir, M.D.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Zhang, K.; Xu, H.H.K. Protein-repellent nanocomposite with rechargeable calcium and phosphate for long-term ion release. Dent. Mater. 2018, 34, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xie, X.; Wang, B.; Weir, M.D.; Oates, T.W.; Xu, H.H.K.; Zhang, N.; Bai, Y. Protein-repellent and antibacterial effects of a novel polymethyl methacrylate resin. J. Dent. 2018, 79, 39–45. [Google Scholar] [CrossRef]
- Choi, W.; Jin, J.; Park, S.; Kim, J.-Y.; Lee, M.-J.; Sun, H.; Kwon, J.-S.; Lee, H.; Choi, S.-H.; Hong, J. Quantitative Interpretation of Hydration Dynamics Enabled the Fabrication of a Zwitterionic Antifouling Surface. ACS Appl. Mater. Interfaces 2020, 12, 7951–7965. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.; Qi, M.; Weir, M.D.; Reynolds, M.A.; Li, C.; Zhou, C.; Xu, H.H.K. Effects of single species versus multispecies periodontal biofilms on the antibacterial efficacy of a novel bioactive Class-V nanocomposite. Dent. Mater. 2019, 35, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, Y.; Weir, M.D.; Lei, L.; Masri, R.; Lynch, C.D.; Oates, T.W.; Zhang, K.; Hu, T.; Xu, H.H.K. Effects of S. mutans gene-modification and antibacterial calcium phosphate nanocomposite on secondary caries and marginal enamel hardness. RSC Adv. 2019, 9, 41672–41683. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, S.; Cheng, L.; Jiang, Y.; Melo, M.A.S.; Weir, M.D.; Oates, T.W.; Zhou, X.; Xu, H.H.K. Novel dental composite with capability to suppress cariogenic species and promote non-cariogenic species in oral biofilms. Mater. Sci. Eng. C 2019, 94, 587–596. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yumoto, H.; Miyamoto, K.; Hirota, K.; Nakae, H.; Tanaka, S.; Murakami, K.; Kudo, Y.; Ozaki, K.; Miyake, Y. 2-Methacryloyloxyethyl phosphorylcholine (MPC)-polymer suppresses an increase of oral bacteria: A single-blind, crossover clinical trial. Clin. Oral Investig. 2019, 23, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Jain, P.; Ma, J.; Smith, J.K.; Yuan, Z.; Hung, H.-C.; He, Y.; Lin, X.; Wu, K.; Pfaendtner, J.; et al. Trimethylamine N-oxide–derived zwitterionic polymers: A new class of ultralow fouling bioinspired materials. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Bonet, A.; Kaufman, G.; Yang, Y.; Wong, C.; Jackson, A.; Huyang, G.; Bowen, R.; Sun, J. Preparation of Dental Resins Resistant to Enzymatic and Hydrolytic Degradation in Oral Environments. Biomacromolecules 2015, 16, 3381–3388. [Google Scholar] [CrossRef] [Green Version]
- Tone, S.; Hasegawa, M.; Puppulin, L.; Pezzotti, G.; Sudo, A. Surface modifications and oxidative degradation in MPC-grafted highly cross-linked polyethylene liners retrieved from short-term total hip arthroplasty. Acta Biomater. 2018, 66, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, E.; Lange, S.C.; Pujari, S.P.; Tijhaar, E.J.; Smulders, M.M.J.; Savelkoul, H.F.J.; Zuilhof, H. Systematic Comparison of Zwitterionic and Non-Zwitterionic Antifouling Polymer Brushes on a Bead-Based Platform. Langmuir 2019, 35, 1181–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, M.; Vegas, A.J.; Anderson, D.G.; Langer, R.S.; Kilduff, J.E.; Belfort, G. Combinatorial synthesis with high throughput discovery of protein-resistant membrane surfaces. Biomaterials 2013, 34, 6133–6138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbrogno, J.; Williams, M.D.; Belfort, G. A New Combinatorial Method for Synthesizing, Screening, and Discovering Antifouling Surface Chemistries. ACS Appl. Mater. Interfaces 2015, 7, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Imbrogno, J.; Belfort, G.; Wang, X.-L. Making polymeric membranes antifouling via “grafting from” polymerization of zwitterions. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Tsao, C.; Liu, S.; Jain, P.; Sinclair, A.; Hung, H.-C.; Bai, T.; Wu, K.; Jiang, S. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc. Natl. Acad. Sci. USA 2015, 112, 12046–12051. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres Jr, L.; Bienek, D.R. Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials. J. Funct. Biomater. 2020, 11, 54. https://doi.org/10.3390/jfb11030054
Torres Jr L, Bienek DR. Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials. Journal of Functional Biomaterials. 2020; 11(3):54. https://doi.org/10.3390/jfb11030054
Chicago/Turabian StyleTorres Jr, Leopoldo, and Diane R. Bienek. 2020. "Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials" Journal of Functional Biomaterials 11, no. 3: 54. https://doi.org/10.3390/jfb11030054