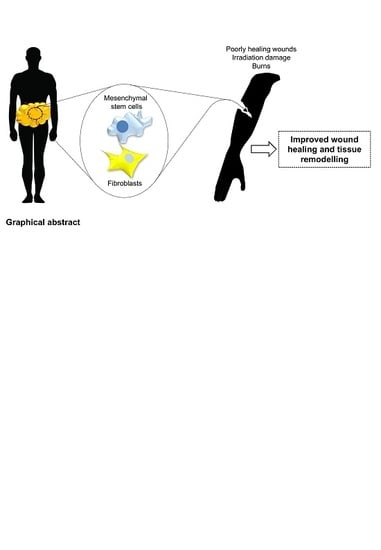
Adipose Tissue: A Source of Stem Cells with Potential for Regenerative Therapies for Wound Healing
Abstract
:1. Introduction
2. Adipose Tissue: Origins, Types, Distribution, and Genetics
3. Wound Healing
4. Adipose Tissue as a Source of Stem Cells
5. Tissue Engineering Techniques for Wound Healing
6. Further Adipose-Derived Targets with Implications for Wound Healing
7. Future Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate protein kinase |
| ASCs | Adipose-derived stem cells |
| α-SMA | Alpha smooth muscle actin |
| BAT | Brown adipose tissue |
| JNK | C-Jun N-terminal kinase |
| C/EBPα | CCAAT-enhancer-binding protein alpha |
| CAL | Cell-assisted lipotransfer |
| CM | Conditioned media |
| dWAT | Dermal white adipose tissue |
| EPCs | Endothelial progenitor cells |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinase |
| FGF | Fibroblast growth factor |
| FDA | Food and Drug Administration |
| HGF | Hepatocyte growth factor |
| ISCT | International Society for Cellular Therapy |
| KGF | Keratinocyte growth factor |
| LAF | Liposuction aspirate fluid |
| lncRNA | Long noncoding RNA |
| MMP | Matrix metalloproteinase |
| MSCs | Mesenchymal stem cells |
| mRNA | Messenger RNA |
| miRNA | MicroRNA |
| NANOG | Nanog homeobox |
| OCT4 | Octamer-binding transcription factor 4 |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PDGF | Platelet-derived growth factor |
| PLA | Processed lipoaspirate |
| SOX2 | Sex-determining region Y-box 2 |
| SVF | Stromal vascular fraction |
| sWAT | Subcutaneous white adipose tissue |
| TIMPs | Tissue inhibitors of matrix metalloproteinases |
| TGFβ | Transforming growth factor beta |
| VEGF | Vascular endothelial growth factor |
| vWAT | Visceral white adipose tissue |
| WAT | White adipose tissue |
References
- Sarantopoulos, C.N.; Banyard, D.; Ziegler, M.E.; Sun, B.; Shaterian, A.; Widgerow, A.D. Elucidating the Preadipocyte and Its Role in Adipocyte Formation: A Comprehensive Review. Stem Cell Rev. Rep. 2017, 14, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- Billon, N.; Iannarelli, P.; Monteiro, M.C.; Glavieux-Pardanaud, C.; Richardson, W.D.; Kessaris, N.; Dani, C.; Dupin, E. The generation of adipocytes by the neural crest. Development 2007, 134, 2283–2292. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1842, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus White Adipose Tissue: A Mini-Review. Gerontology 2012, 58, 15–23. [Google Scholar] [CrossRef]
- Driskell, R.R.; Jahoda, C.A.B.; Chuong, C.-M.; Watt, F.M.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2014, 23, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Kruglikov, I.L.; Scherer, P.E. Dermal Adipocytes: From Irrelevance to Metabolic Targets? Trends Endocrinol. Metab. 2015, 27, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Arda, O.; Goksugur, N.; Tüzün, Y.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissebah, A.H.; Krakower, G.R. Regional adiposity and morbidity. Physiol. Rev. 1994, 74, 761–811. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.L.; Brandon, D.T.; Wiggins, S.A.; Whitfield, K.E. Genetic and Environmental Influences on Body-Fat Measures among African-American Twins. Obes. Res. 2002, 10, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Schleinitz, R.; Böttcher, Y.; Blüher, M.; Kovács, P. The genetics of fat distribution. Diabetology 2014, 57, 1276–1286. [Google Scholar] [CrossRef]
- Tibbs, M.K. Wound healing following radiation therapy: A review. Radiother. Oncol. 1997, 42, 99–106. [Google Scholar] [CrossRef]
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2012, 70, 2059–2081. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.J.; Clark, R.A. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Yarnold, J.R.; Brotons, M.-C.V. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef]
- Evans, N.D.; Oreffo, R.O.; Healy, E.; Thurner, P.J.; Man, Y.H. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 2013, 28, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Gieringer, M.; Gosepath, J.; Naim, R. Radiotherapy and wound healing: Principles, management and prospects (Review). Oncol. Rep. 2011, 26, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, C.; Toki, F.; Shiraishi, K.; Sayama, K.; Nishimura, E.K.; Miura, H.; Higashiyama, S.; Nanba, D. Two clonal types of human skin fibroblasts with different potentials for proliferation and tissue remodeling ability. J. Dermatol. Sci. 2016, 82, 84–94. [Google Scholar] [CrossRef]
- Pittenger, M.F. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Oron, E.; Nelson, B.; Razis, S.; Ivanova, N. Distinct Lineage Specification Roles for NANOG, OCT4, and SOX2 in Human Embryonic Stem Cells. Cell Stem Cell 2012, 10, 440–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitrone, M.; Pizzolanti, G.; Coppola, A.; Tomasello, L.; Martorana, S.; Pantuso, G.; Giordano, C. Knockdown of NANOG Reduces Cell Proliferation and Induces G0/G1 Cell Cycle Arrest in Human Adipose Stem Cells. Int. J. Mol. Sci. 2019, 20, 2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation☆. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White Fat Progenitor Cells Reside in the Adipose Vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.A.; Zhang, F.; Jiang, L.; Ye, R.; An, Y.A.; Shao, M.; Tao, C.; Gupta, R.K.; Scherer, P.E. Peroxisome Proliferator-Activated Receptor γ and Its Role in Adipocyte Homeostasis and Thiazolidinedione-Mediated Insulin Sensitization. Mol. Cell. Biol. 2018, 38, e00677-17. [Google Scholar] [CrossRef] [Green Version]
- Schipper, B.M.; Marra, K.G.; Zhang, W.; Donnenberg, A.D.; Rubin, J.P. Regional Anatomic and Age Effects on Cell Function of Human Adipose-Derived Stem Cells. Ann. Plast. Surg. 2008, 60, 538–544. [Google Scholar] [CrossRef]
- Reumann, M.K.; Linnemann, C.; Aspera-Werz, R.H.; Arnold, S.; Held, M.; Seeliger, C.; Nussler, A.K.; Ehnert, S. Donor Site Location Is Critical for Proliferation, Stem Cell Capacity, and Osteogenic Differentiation of Adipose Mesenchymal Stem/Stromal Cells: Implications for Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1868. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, S.; Cantini, G.; Poli, G.; Francalanci, M.; Squecco, R.; Di Franco, A.; Borgogni, E.; Frontera, S.; Nesi, G.; Liotta, F.; et al. Functional Differences in Visceral and Subcutaneous Fat Pads Originate from Differences in the Adipose Stem Cell. PLoS ONE 2012, 7, e36569. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Niklason, L.; Steinbacher, D. The Effect of Age on Human Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2013, 131, 27–37. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Rodbell, M. Metabolism of isolated fat cells. II. The similar effects of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J. Biol. Chem. 1966, 241, 130–139. [Google Scholar] [PubMed]
- Chen, Y.-J.; Liu, H.-Y.; Chang, Y.-T.; Cheng, Y.-H.; Mersmann, H.J.; Kuo, W.-H.; Ding, S.-T. Isolation and Differentiation of Adipose-Derived Stem Cells from Porcine Subcutaneous Adipose Tissues. J. Vis. Exp. 2016, 109, e53886. [Google Scholar] [CrossRef] [Green Version]
- Oedayrajsingh-Varma, M.; Van Ham, S.; Knippenberg, M.; Helder, M.; Klein-Nulend, J.; Schouten, T.; Ritt, M.; Van Milligen, F. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006, 8, 166–177. [Google Scholar] [CrossRef]
- Johnson, M.L.; Johnson, L.; Mahabir, R.C.; Bernard, R. Perspectives on the FDA Draft Guidances for Use of Adipose Tissue. Aesthetic Surg. J. 2017, 37, 622–625. [Google Scholar] [CrossRef] [Green Version]
- Rigotti, G.; Marchi, A.; Galie’, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical Treatment of Radiotherapy Tissue Damage by Lipoaspirate Transplant: A Healing Process Mediated by Adipose-Derived Adult Stem Cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef]
- Sharma, S.; Rahul, M. Role of autologous fat grafting in burn wounds. Indian J. Burn. 2018, 26, 15. [Google Scholar] [CrossRef]
- Riyat, H.; Touil, L.L.; Briggs, M.; Shokrollahi, K. Autologous fat grafting for scars, healing and pain: A review. Scars Burns Heal. 2017, 3, 205951311772820. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Deng, Y.-H.; Tsai, F.-C.; Deng, W.-P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef] [Green Version]
- Bhang, S.H.; Cho, S.-W.; La, W.-G.; Lee, T.-J.; Yang, H.S.; Sun, A.-Y.; Baek, S.-H.; Rhie, J.-W.; Kim, B.-S. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 2011, 32, 2734–2747. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Wang, C.; Patel, R.; Trujillo, A.; Patel, N.A.; Prather, J.; Gould, L.J.; Wu, M.H. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv. Wound Care 2018, 7, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Makino, T.; Jinnin, M.; Muchemwa, F.; Fukushima, S.; Kogushi-Nishi, H.; Moriya, C.; Igata, T.; Fujisawa, A.; Johno, T.; Ihn, H. Basic fibroblast growth factor stimulates the proliferation of human dermal fibroblasts via the ERK1/2 and JNK pathways. Br. J. Dermatol. 2009, 162, 717–723. [Google Scholar] [CrossRef]
- Yuan, B.; Broadbent, J.A.; Huan, J.; Yang, H. The Effects of Adipose Stem Cell-Conditioned Media on Fibrogenesis of Dermal Fibroblasts Stimulated by Transforming Growth Factor-β1. J. Burn Care Res. 2018, 39, 129–140. [Google Scholar] [CrossRef]
- Zvonic, S.; Lefevre, M.; Kilroy, G.; Floyd, Z.E.; Delany, J.P.; Kheterpal, I.; Gravois, A.; Dow, R.; White, A.; Wu, X.; et al. Secretome of Primary Cultures of Human Adipose-derived Stem Cells. Mol. Cell. Proteom. 2006, 6, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Ezure, T.; Amano, S. Adiponectin and leptin up-regulate extracellular matrix production by dermal fibroblasts. BioFactors 2007, 31, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, J.; Su, L.; Cheng, S.; Zhou, J.; Ye, X.; Dong, Y.; Sun, S.; Qi, F.-Z.; Liu, Z.; et al. Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burn 2018, 44, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laberge, A.; Arif, S.; Moulin, V. Microvesicles: Intercellular messengers in cutaneous wound healing. J. Cell. Physiol. 2018, 233, 5550–5563. [Google Scholar] [CrossRef] [PubMed]
- Clancy, S.; Brown, W. Translation: DNA to mRNA to Protein. Nat. Educ. 2008, 1, 101. [Google Scholar]
- Pogue, A.I.; Clement, C.; Hill, J.M.; Lukiw, W.J. Evolution of microRNA (miRNA) Structure and Function in Plants and Animals: Relevance to Aging and Disease. J. Aging Sci. 2014, 2, 119. [Google Scholar] [CrossRef] [Green Version]
- Yao, R.; Wang, Y.; Chen, L.-L. Cellular functions of long noncoding RNAs. Nature 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, T.; Pouzoulet, F.; Squiban, C.; Buard, V.; André, M.; Cousin, B.; Gourmelon, P.; Benderitter, M.; Casteilla, L.; Tamarat, R. Cell Therapy Based on Adipose Tissue-Derived Stromal Cells Promotes Physiological and Pathological Wound Healing. Arter. Thromb. Vasc. Biol. 2009, 29, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, A.M.; Yan, Y.; Matthias, N.; Bai, X.; Rios, C.; Mathur, A.; Song, Y.-H.; Alt, E.U. IFATS Collection: Human Adipose-Derived Stem Cells Seeded on a Silk Fibroin-Chitosan Scaffold Enhance Wound Repair in a Murine Soft Tissue Injury Model. Stem Cells 2009, 27, 250–258. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, J.-W.; Jun, H.-S.; Roh, J.Y.; Lee, H.-Y.; Hong, I.-S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2017, 26, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.A.; Horsley, V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 2013, 140, 1517–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hattab, M.Y.; Nagumo, Y.; Gourronc, F.A.; Klingelhutz, A.J.; Ankrum, J.A.; Sander, E.A. Human Adipocyte Conditioned Medium Promotes In Vitro Fibroblast Conversion to Myofibroblasts. Sci. Rep. 2020, 10, 10. [Google Scholar] [CrossRef]
- Zhou, P.; Deng, C.-R.; Ping, J.-L. Identification of Y (4008), Y (4140), Y (4260), and Y (4360) as Tetraquark States. Chin. Phys. Lett. 2015, 32, 101201. [Google Scholar] [CrossRef]
- Haydont, V.; Neiveyans, V.; Perez, P.; Busson, É.; Lataillade, J.-J.; Asselineau, D.; Fortunel, N.O. Fibroblasts from the Human Skin Dermo-Hypodermal Junction are Distinct from Dermal Papillary and Reticular Fibroblasts and from Mesenchymal Stem Cells and Exhibit a Specific Molecular Profile Related to Extracellular Matrix Organization and Modeling. Cells 2020, 9, 368. [Google Scholar] [CrossRef] [Green Version]
- Pierpont, Y.N.; Dinh, T.P.; Salas, R.E.; Johnson, E.L.; Wright, T.G.; Robson, M.C.; Payne, W.G. Obesity and Surgical Wound Healing: A Current Review. Int. Sch. Res. Not. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, S.; Tada, Y.; Asano, Y.; Hau, C.S.; Kato, T.; Saeki, H.; Yamauchi, T.; Kubota, N.; Kadowaki, T.; Sato, S. Adiponectin Regulates Cutaneous Wound Healing by Promoting Keratinocyte Proliferation and Migration via the ERK Signaling Pathway. J. Immunol. 2012, 189, 3231–3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Stimulatory Effect of ASC Secretome on Dermal Fibroblast Function | Paracrine Factors Secreted by ASCs | References |
|---|---|---|
| Increased proliferation | FGF-2 | [45,46,60] |
| HGF | [60] | |
| Exosomes | [56,61] | |
| Microvesicles | [57] | |
| Increased migration | FGF-2 | [45,46] |
| Exosomes | [45,56,61] | |
| Microvesicles | [57] | |
| Matrix Remodeling (increased production of collagen I and III, TGFβ, FGF2, and MMP1) | Adiponectin | [49] |
| Exosomes | [56,61] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trevor, L.V.; Riches-Suman, K.; Mahajan, A.L.; Thornton, M.J. Adipose Tissue: A Source of Stem Cells with Potential for Regenerative Therapies for Wound Healing. J. Clin. Med. 2020, 9, 2161. https://doi.org/10.3390/jcm9072161
Trevor LV, Riches-Suman K, Mahajan AL, Thornton MJ. Adipose Tissue: A Source of Stem Cells with Potential for Regenerative Therapies for Wound Healing. Journal of Clinical Medicine. 2020; 9(7):2161. https://doi.org/10.3390/jcm9072161
Chicago/Turabian StyleTrevor, Lucy V, Kirsten Riches-Suman, Ajay L Mahajan, and M Julie Thornton. 2020. "Adipose Tissue: A Source of Stem Cells with Potential for Regenerative Therapies for Wound Healing" Journal of Clinical Medicine 9, no. 7: 2161. https://doi.org/10.3390/jcm9072161