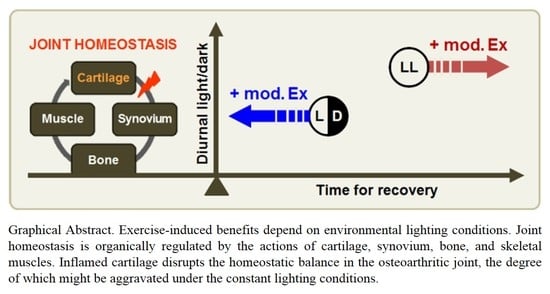
Conditional Controlled Light/Dark Cycle Influences Exercise-Induced Benefits in a Rat Model with Osteoarthritis: In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Induction of Osteoarthritis and Exercise Training
2.2. Behavioral Assessments
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Histomorphological Assessments
2.5. Primary Cell Isolation and Culture
2.6. TRAP Staining
2.7. Quantitative Real-Time PCR Analysis
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Continuous Lighting Induces an Inflammatory Microenvironment in Osteoarthritic Joints
3.2. Continuous Light Exposure is Injurious to Bone Cells
3.3. Aberrant Lighting Conditions Causes a Reduction in Exercise-Induced Benefits in Inflamed Joints
3.4. Exercise Training under an Abnormal Light/Dark Cycle Facilitates Muscular Inflammation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, K.R.; Golightly, Y.M. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Pract. Res. Clin. Rheumatol. 2015, 29, 120–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhl, C.; Christensen, R.; Roos, E.M.; Zhang, W.; Lund, H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: A systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014, 66, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Benatti, F.B.; Pedersen, B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef]
- Daenen, L.; Varkey, E.; Kellmann, M.; Nijs, J. Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying science to practice. Clin. J. Pain 2015, 31, 108–114. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Valdes, K.; Pedersini, P.; Berjano, P. Osteoarthritis: A call for research on central pain mechanism and personalized prevention strategies. Clin. Rheumatol. 2019, 38, 583–584. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Bishop, M.D.; Pedersini, P.; Berjano, P. Physical activity and osteoarthritis: Update and perspectives. Pain Med. 2019, 20, 1461–1463. [Google Scholar] [CrossRef]
- Leong, D.J.; Sun, H.B. Osteoarthritis—Why exercise? J. Exerc. Sports Orthop. 2014, 1, 04. [Google Scholar]
- Georgiev, T.; Angelov, A.K. Modifiable risk factors in knee osteoarthritis: Treatment implications. Rheumatol. Int. 2019, 39, 1145–1157. [Google Scholar] [CrossRef]
- Jones, P.R.; Barton, C.; Morrissey, D.; Maffulli, N.; Hemmings, S. Pre-cooling for endurance exercise performance in the heat: A systematic review. BMC Med. 2012, 10, 166. [Google Scholar] [CrossRef]
- West, A.C.; Smith, L.; Ray, D.W.; Loudon, A.S.I.; Brown, T.M.; Bechtold, D.A. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat. Commun. 2017, 8, 417. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Nelson, R.J. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 2017, 7, e1017. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Hong, Y.; Jin, Y.; Park, K.; Choi, J.; Kang, H.; Lee, S.R.; Hong, Y. Elevated serum melatonin under constant darkness enhances neural repair in spinal cord injury through regulation of circadian clock proteins expression. J. Clin. Med. 2019, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Charmandari, E.; Kino, T.; Chrousos, G.P. Stress-related and circadian secretion and target tissue actions of glucocorticoids: Impact on health. Front. Endocrinol. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Botter, S.M.; van Osch, G.J.; Waarsing, J.H.; van der Linden, J.C.; Verhaar, J.A.; Pols, H.A.; van Leeuwen, J.P.; Weinans, H. Cartilage damage pattern in relation to subchondral plate thickness in a collagenase-induced model of osteoarthritis. Osteoarthr. Cartil. 2008, 16, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, H.; Lee, S.; Jin, Y.; Choi, J.; Lee, S.R.; Chang, K.T.; Hong, Y. Role of melatonin combined with exercise as a switch-like regulator for circadian behavior in advanced osteoarthritic knee. Oncotarget 2017, 8, 97633–97647. [Google Scholar] [CrossRef] [Green Version]
- Bakker, A.D.; Klein-Nulend, J. Osteoblast isolation from murine calvaria and long bones. Methods Mol. Biol. 2012, 816, 19–29. [Google Scholar]
- Orriss, I.R.; Taylor, S.E.; Arnett, T.R. Rat osteoblast cultures. Methods Mol. Biol. 2012, 816, 31–41. [Google Scholar]
- Xing, L.; Boyce, B.F. Rankl-based osteoclastogenic assays from murine bone marrow cells. Methods Mol. Biol. 2014, 1130, 307–313. [Google Scholar] [PubMed]
- Oishi, K.; Higo-Yamamoto, S.; Yamamoto, S.; Yasumoto, Y. Disrupted light-dark cycle abolishes circadian expression of peripheral clock genes without inducing behavioral arrhythmicity in mice. Biochem. Biophys. Res. Commun. 2015, 458, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Abilio, V.C.; Freitas, F.M.; Dolnikoff, M.S.; Castrucci, A.M.; Frussa-Filho, R. Effects of continuous exposure to light on behavioral dopaminergic supersensitivity. Biol. Psychiatry 1999, 45, 1622–1629. [Google Scholar] [CrossRef]
- Cernysiov, V.; Gerasimcik, N.; Mauricas, M.; Girkontaite, I. Regulation of t-cell-independent and t-cell-dependent antibody production by circadian rhythm and melatonin. Int. Immunol. 2010, 22, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.; Rajaratnam, S.M.; Van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011, 96, E463–E472. [Google Scholar] [CrossRef]
- Fukuhara, C.; Aguzzi, J.; Bullock, N.; Tosini, G. Effect of long-term exposure to constant dim light on the circadian system of rats. Neurosignals 2005, 14, 117–125. [Google Scholar] [CrossRef]
- Seifman, M.A.; Adamides, A.A.; Nguyen, P.N.; Vallance, S.A.; Cooper, D.J.; Kossmann, T.; Rosenfeld, J.V.; Morganti-Kossmann, M.C. Endogenous melatonin increases in cerebrospinal fluid of patients after severe traumatic brain injury and correlates with oxidative stress and metabolic disarray. J. Cereb. Blood Flow Metab. 2008, 28, 684–696. [Google Scholar] [CrossRef]
- Dagnino-Subiabre, A.; Orellana, J.A.; Carmona-Fontaine, C.; Montiel, J.; Diaz-Veliz, G.; Seron-Ferre, M.; Wyneken, U.; Concha, M.L.; Aboitiz, F. Chronic stress decreases the expression of sympathetic markers in the pineal gland and increases plasma melatonin concentration in rats. J. Neurochem. 2006, 97, 1279–1287. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis. Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef]
- Adaes, S.; Mendonca, M.; Santos, T.N.; Castro-Lopes, J.M.; Ferreira-Gomes, J.; Neto, F.L. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Arthritis Res. Ther. 2014, 16, R10. [Google Scholar] [CrossRef]
- Maestroni, G.J.; Cardinali, D.P.; Esquifino, A.I.; Pandi-Perumal, S.R. Does melatonin play a disease-promoting role in rheumatoid arthritis? J. Neuroimmunol. 2005, 158, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Chang, H.W.; Jung, H.R.; Cho, C.H.; Hur, J.A.; Lee, S.I.; Choi, T.H.; Kim, S.H.; Ha, E. Melatonin attenuates clock gene cryptochrome1, which may aggravate mouse anti-type II collagen antibody-induced arthritis. Rheumatol. Int. 2012, 32, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Caliani, A.J.; Jimenez-Jorge, S.; Molinero, P.; Guerrero, J.M.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Osuna, C. Dual effect of melatonin as proinflammatory and antioxidant in collagen-induced arthritis in rats. J. Pineal Res. 2005, 38, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef]
- Qin, B.; Deng, Y. Overexpression of circadian clock protein cryptochrome (cry) 1 alleviates sleep deprivation-induced vascular inflammation in a mouse model. Immunol. Lett. 2015, 163, 76–83. [Google Scholar] [CrossRef]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Liu, F.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef] [Green Version]
- Hashiramoto, A.; Yamane, T.; Tsumiyama, K.; Yoshida, K.; Komai, K.; Yamada, H.; Yamazaki, F.; Doi, M.; Okamura, H.; Shiozawa, S. Mammalian clock gene cryptochrome regulates arthritis via proinflammatory cytokine tnf-alpha. J. Immunol. 2010, 184, 1560–1565. [Google Scholar] [CrossRef]
- Kiessling, S.; Sollars, P.J.; Pickard, G.E. Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus. PLoS ONE 2014, 9, e92959. [Google Scholar] [CrossRef]
- Park, S.Y.; Walker, J.J.; Johnson, N.W.; Zhao, Z.; Lightman, S.L.; Spiga, F. Constant light disrupts the circadian rhythm of steroidogenic proteins in the rat adrenal gland. Mol. Cell Endocrinol. 2013, 371, 114–123. [Google Scholar] [CrossRef]
- Tapia-Osorio, A.; Salgado-Delgado, R.; Angeles-Castellanos, M.; Escobar, C. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav. Brain Res. 2013, 252, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue-specific actions of glucocorticoids. J. Biol. Chem. 2011, 286, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nievas, B.G.; Garcia-Bueno, B.; Caso, J.R.; Menchen, L.; Leza, J.C. Corticosterone as a marker of susceptibility to oxidative/nitrosative cerebral damage after stress exposure in rats. Psychoneuroendocrinology 2007, 32, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J. Neurohormonal-cytokine interactions: Implications for inflammation, common human diseases and well-being. Neurochem. Int. 2008, 52, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Monsalves-Alvarez, M.; Henriquez, S.; Llanos, M.N.; Troncoso, R. Glucocorticoid resistance in chronic diseases. Steroids 2016, 115, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Seitz, S.; Baschant, U.; Schilling, A.F.; Illing, A.; Stride, B.; Kirilov, M.; Mandich, V.; Takacz, A.; Schmidt-Ullrich, R.; et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010, 11, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Glucocorticoids and the osteoclast. Clin. Exp. Rheumatol. 2015, 33, S37–S39. [Google Scholar]
- Novack, D.V. Editorial: Inflammatory osteoclasts: A different breed of bone eaters? Arthritis Rheumatol. 2016, 68, 2834–2836. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Karatzas, D.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 2009, 15, 208–216. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, G.; Zhu, G.; Ma, C.; Zhao, H. Chronic sleep restriction induces changes in the mandibular condylar cartilage of rats: Roles of Akt, Bad and Caspase-3. Int. J. Clin. Exp. Med. 2014, 7, 2585–2592. [Google Scholar]





| Gene | Primer Sequence (5′-3′) | Size (bp) | GenBank Accession No. |
|---|---|---|---|
| Actb | F: taa aga cct cta tgc caa cac agt R: cac gat gga ggg gcc gga ctc atc | 241 | NM_031144.2 |
| Gapdh | F: ctc agt tgc tga gga gtc cc R: att cga gag aag gga ggg ct | 120 | NM_017008.4 |
| Adamts4 | F: agc ctt taa gca tcc aag ca R: gga ggg ttt agg cct ttc tg | 153 | NM_023959.1 |
| Adrb2 | F: aca cgg gaa tga cag cga ctt c R: cga tcc act gca atc acg cac | 384 | NM_012492.2 |
| Bglap | F: tcc aag cag gag ggc agt aag R: taa acg gtg gtg cca tag atg c | 194 | NM_013414.1 |
| Bmal1 | F: gtc gaa tga ttg ccg agg aa R: ggg agg cgt act tgt gat gtt c | 101 | AB015203 |
| Col1a1 | F: tct gac tgg aag agc gga gag R: gag tgg gga aca cac agg tct | 112 | NM_053304.1 |
| Col2a1 | F: ggt ttg gag aga cca tga acg g R: gtc aac aat ggg aag gcg tga g | 350 | NM_012929.1 |
| Cry1 | F: gcc tca gtc cct tct aat cc R: tcc cgc atg ctt tcg tat c | 284 | NM_198750.2 |
| Ctsk | F: ctg gga gac atg acc agc gaa g R: tgc act tag ctg cct ttg cc | 433 | NM_031560.2 |
| Dcstamp | F: tgc aac cta agg gca aag agc R: gag gcc agt gct gac tag gat g | 309 | NM_029422.4 |
| Fbxo32 | F: gca aaa cat aag act cat acg R: gta gag tgg tct cca ttc g | 134 | NM_133521.1 |
| Fndc5 | F: ctc agc aga aga agg atg tga g R: cat ggt cac ctc atc ttt gtt c | 221 | NM_001270981.1 |
| Igf1 | F: cgc tct tca gtt cgt gtg tg R: cgg aag caa cac tca tcc ac | 114 | NM_001082477.2 |
| Il1b | F: aac aaa aat gcc tcg tgc tg R: ttg tcg ttg ctt gtc tct cc | 124 | NM_031512.2 |
| Il6 | F: cac aga gga tac cac cca ca R: cac aaa ctc cag gta gaa acg g | 277 | NM_012589.2 |
| Il10 | F: taa ctg cac cca ctt ccc agt c R: cat tct tca cct gct cca ctg c | 350 | NM_012854.2 |
| Mmp3 | F: tga gag cag tgc aga act gtg g R: ctt gtg cat cag ctc cat agt g | 296 | NM_133523.3 |
| Mmp9 | F: gct atg gtt aca ctc ggg ca R: tgg cct tta gtg tct cgc tg | 129 | NM_031055.1 |
| Mmp13 | F: agg cct tca gaa aag cct tc R: gag ctg ctt gtc cag gtt tc | 226 | NM_133530.1 |
| Mstn | F: gct ggc cca gtg gat cta aat g R: tga ttg ttt ccg tgg tag cgt g | 304 | NM_019151.1 |
| Sost | F: gta cat gca gcc ttc gtt gct g R: act ggt tgt gga agc ggg tga g | 490 | NM_030584.1 |
| Sox9 | F: aca acg caa gct tct gca ag R: aca ctc tcc aac cac agc ag | 111 | NM_080403.1 |
| Timp3 | F: ggc caa agt ggt ggg aaa gaa g R: ccc acc tct cca caa agt tgc | 245 | NM_012886.2 |
| Tnfa | F: cta ctg aac ttc ggg gtg atc R: ctt gtc cct tga aga gaa cct g | 292 | NM_012675.3 |
| Trap | F: gat cac ctt ggc aat gtc tcg R: ggc tga caa agt cgt cgg aat | 175 | NM_019144.2 |
| Trim63 | F: agg tga agg agg aac tga g R: aac tgc tct cgg tac tgg | 148 | NM_080903.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Lee, S.; Choi, J.; Jin, Y.; Won, J.; Jo, Y.; Hong, Y. Conditional Controlled Light/Dark Cycle Influences Exercise-Induced Benefits in a Rat Model with Osteoarthritis: In Vitro and In Vivo Study. J. Clin. Med. 2019, 8, 1855. https://doi.org/10.3390/jcm8111855
Hong Y, Lee S, Choi J, Jin Y, Won J, Jo Y, Hong Y. Conditional Controlled Light/Dark Cycle Influences Exercise-Induced Benefits in a Rat Model with Osteoarthritis: In Vitro and In Vivo Study. Journal of Clinical Medicine. 2019; 8(11):1855. https://doi.org/10.3390/jcm8111855
Chicago/Turabian StyleHong, Yunkyung, Seunghoon Lee, Jeonghyun Choi, Yunho Jin, Jinyoung Won, Youngjin Jo, and Yonggeun Hong. 2019. "Conditional Controlled Light/Dark Cycle Influences Exercise-Induced Benefits in a Rat Model with Osteoarthritis: In Vitro and In Vivo Study" Journal of Clinical Medicine 8, no. 11: 1855. https://doi.org/10.3390/jcm8111855