Proteomic Analysis of Longitudinal Changes in Blood Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
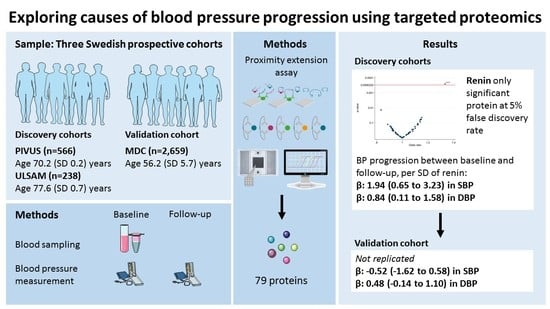
2.1.1. The Prospective Investigation of the Vasculature in Uppsala Seniors Study (PIVUS)
2.1.2. Uppsala Longitudinal Study of Adult Men (ULSAM)
2.1.3. The Malmö Diet and Cancer Study (MDC)
2.2. Ethical Considerations
2.3. Baseline and Follow-Up Investigations
2.4. Proteomic Profiling
2.5. Sample Size Estimation
2.6. Statistical Analysis
3. Results
3.1. Discovery–Validation Phase
3.2. Best Estimates Phase
3.3. Causality Phase
4. Discussion
4.1. Principal Observations
4.2. Previous Proteomics Studies in Hypertension
4.3. Previously Studied Proteins Related to Hypertension
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W., Jr. The genetic dissection of essential hypertension. Nat. Rev. Genet. 2006, 7, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Delles, C.; McBride, M.W.; Graham, D.; Padmanabhan, S.; Dominiczak, A.F. Genetics of hypertension: From experimental animals to humans. Biochim. Biophys. Acta 2010, 1802, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Iiyama, K.; Nagano, M.; Yo, Y.; Nagano, N.; Kamide, K.; Higaki, J.; Mikami, H.; Ogihara, T. Impaired endothelial function with essential hypertension assessed by ultrasonography. Am. Heart J. 1996, 132, 779–782. [Google Scholar] [CrossRef]
- Yannoutsos, A.; Levy, B.I.; Safar, M.E.; Slama, G.; Blacher, J. Pathophysiology of hypertension: Interactions between macro and microvascular alterations through endothelial dysfunction. J. Hypertens. 2014, 32, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.H.; Wachtell, K.; Aalkjaer, C.; Dige-Petersen, H.; Rokkedal, J.; Ibsen, H. Vasodilatory capacity and vascular structure in long-standing hypertension: A life substudy. Losartan intervention for endpoint-reduction in hypertension. Am. J. Hypertens. 2002, 15, 398–404. [Google Scholar] [CrossRef]
- Mulvany, M.J. Vascular remodelling of resistance vessels: Can we define this? Cardiovasc. Res. 1999, 41, 9–13. [Google Scholar] [CrossRef]
- Shimbo, D.; Muntner, P.; Mann, D.; Barr, R.G.; Tang, W.; Post, W.; Lima, J.; Burke, G.; Bluemke, D.; Shea, S. Association of left ventricular hypertrophy with incident hypertension: The multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2011, 173, 898–905. [Google Scholar] [CrossRef]
- De Simone, G.; Devereux, R.B.; Chinali, M.; Roman, M.J.; Welty, T.K.; Lee, E.T.; Howard, B.V. Left ventricular mass and incident hypertension in individuals with initial optimal blood pressure: The strong heart study. J. Hypertens. 2008, 26, 1868–1874. [Google Scholar] [CrossRef]
- Mark, A.L. The sympathetic nervous system in hypertension: A potential long-term regulator of arterial pressure. J. Hypertens. Suppl. 1996, 14, S159–S165. [Google Scholar]
- Hering, D.; Narkiewicz, K. Sympathetic nervous system and arterial hypertension: New perspectives, new data. Kardiologia Polska 2013, 71, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; Galletti, F.; Barba, G. Altered renal handling of sodium in human hypertension: Short review of the evidence. Hypertension 2003, 41, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Cappuccio, F.P.; Iacone, R.; Russo, O.; Galletti, F.; Strazzullo, P. Altered renal sodium handling and risk of incident hypertension: Results of the olivetti heart study. PLoS ONE 2017, 12, e0171973. [Google Scholar] [CrossRef] [PubMed]
- Poli, K.A.; Tofler, G.H.; Larson, M.G.; Evans, J.C.; Sutherland, P.A.; Lipinska, I.; Mittleman, M.A.; Muller, J.E.; D’Agostino, R.B.; Wilson, P.W.; et al. Association of blood pressure with fibrinolytic potential in the framingham offspring population. Circulation 2000, 101, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Wall, U.; Jern, C.; Bergbrant, A.; Jern, S. Enhanced levels of tissue-type plasminogen activator in borderline hypertension. Hypertension 1995, 26, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yeh, F.; de Simone, G.; Best, L.G.; Lee, E.T.; Howard, B.V.; Zhao, J. Relationship between plasma plasminogen activator inhibitor-1 and hypertension in american indians: Findings from the strong heart study. J. Hypertens. 2017, 35, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Rohde, L.E.; Hennekens, C.H.; Ridker, P.M. Survey of c-reactive protein and cardiovascular risk factors in apparently healthy men. Am. J. Cardiol. 1999, 84, 1018–1022. [Google Scholar] [CrossRef]
- Bermudez, E.A.; Rifai, N.; Buring, J.; Manson, J.E.; Ridker, P.M. Interrelationships among circulating interleukin-6, c-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1668–1673. [Google Scholar] [CrossRef]
- Mattace-Raso, F.U.; Verwoert, G.C.; Hofman, A.; Witteman, J.C. Inflammation and incident-isolated systolic hypertension in older adults: The rotterdam study. J. Hypertens. 2010, 28, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Hovind, P.; Schalkwijk, C.G.; Parving, H.H.; Stehouwer, C.D.A.; Rossing, P. Biomarkers of inflammation and endothelial dysfunction as predictors of pulse pressure and incident hypertension in type 1 diabetes: A 20 year life-course study in an inception cohort. Diabetologia 2018, 61, 231–241. [Google Scholar] [CrossRef]
- Solak, Y.; Afsar, B.; Vaziri, N.D.; Aslan, G.; Yalcin, C.E.; Covic, A.; Kanbay, M. Hypertension as an autoimmune and inflammatory disease. Hypertens. Res. 2016, 39, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, G.; Pirro, M.; Gemelli, F.; Pasqualini, L.; Vaudo, G.; Marchesi, S.; Siepi, D.; Bagaglia, F.; Mannarino, E. Increased c-reactive protein concentrations in never-treated hypertension: The role of systolic and pulse pressures. J. Hypertens. 2003, 21, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Suh, J.Y.; Kim, B.S.; Kang, J.H.; Kim, H.; Lee, M.H.; Park, J.R.; Kim, S.W. High sensitivity c-reactive protein as an independent risk factor for essential hypertension. Am. J. Hypertens. 2003, 16, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Lacy, F.; O’Connor, D.T.; Schmid-Schonbein, G.W. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J. Hypertens. 1998, 16, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Gona, P.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Tofler, G.H.; Jacques, P.F.; Meigs, J.B.; Rifai, N.; Selhub, J.; et al. Multiple biomarkers and the risk of incident hypertension. Hypertension 2007, 49, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Rifai, N.; Blake, G.J.; Gaziano, J.M.; Ridker, P.M. C-reactive protein and the risk of developing hypertension. JAMA 2003, 290, 2945–2951. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Wang, L.; Buring, J.E.; Ridker, P.M.; Gaziano, J.M. Comparison of interleukin-6 and c-reactive protein for the risk of developing hypertension in women. Hypertension 2007, 49, 304–310. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Hsu, P.F.; Chang, H.Y.; Bai, C.H.; Yeh, W.T.; Pan, H.W. C-reactive protein predicts systolic blood pressure and pulse pressure but not diastolic blood pressure: The cardiovascular disease risk factors two-township study. Am. J. Hypertens. 2013, 26, 657–664. [Google Scholar] [CrossRef]
- Lakoski, S.G.; Herrington, D.M.; Siscovick, D.M.; Hulley, S.B. C-reactive protein concentration and incident hypertension in young adults: The cardia study. Arch. Intern. Med. 2006, 166, 345–349. [Google Scholar] [CrossRef]
- Vasan, R.S.; Evans, J.C.; Larson, M.G.; Wilson, P.W.; Meigs, J.B.; Rifai, N.; Benjamin, E.J.; Levy, D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N. Engl. J. Med. 2004, 351, 33–41. [Google Scholar] [CrossRef]
- Sesso, H.D.; Jimenez, M.C.; Wang, L.; Ridker, P.M.; Buring, J.E.; Gaziano, J.M. Plasma inflammatory markers and the risk of developing hypertension in men. J. Am. Heart Assoc. 2015, 4, e001802. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Gaziano, J.M.; Liu, S.; Cochrane, B.; Cook, N.R.; Ridker, P.M.; Rifai, N.; Sesso, H.D. Circulating inflammatory and endothelial markers and risk of hypertension in white and black postmenopausal women. Clin. Chem. 2011, 57, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Davey Smith, G.; Lawlor, D.A.; Harbord, R.; Timpson, N.; Rumley, A.; Lowe, G.D.; Day, I.N.; Ebrahim, S. Association of c-reactive protein with blood pressure and hypertension: Life course confounding and mendelian randomization tests of causality. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Sundstrom, J.; Stenemo, M.; Hagstrom, E.; Arnlov, J. Discovery of new biomarkers for atrial fibrillation using a custom-made proteomics chip. Heart 2017, 103, 377–382. [Google Scholar] [CrossRef]
- Nowak, C.; Sundstrom, J.; Gustafsson, S.; Giedraitis, V.; Lind, L.; Ingelsson, E.; Fall, T. Protein biomarkers for insulin resistance and type 2 diabetes risk in two large community cohorts. Diabetes 2016, 65, 276–284. [Google Scholar] [CrossRef]
- Lind, L.; Fors, N.; Hall, J.; Marttala, K.; Stenborg, A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: The prospective investigation of the vasculature in uppsala seniors (pivus) study. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- The PIVUS study. Available online: www.medsci.uu.se/pivus (accessed on 8 August 2019).
- Hedstrand, H. A study of middle-aged men with particular reference to risk factors for cardiovascular disease. Upsala J. Med. Sci. Suppl. 1975, 19, 1–61. [Google Scholar]
- The ULSAM study. Available online: www.pubcare.uu.se/ULSAM (accessed on 8 August 2019).
- Berglund, G.; Elmstahl, S.; Janzon, L.; Larsson, S.A. The malmo diet and cancer study. Design and feasibility. J. Intern. Med. 1993, 233, 45–51. [Google Scholar] [CrossRef]
- Rosvall, M.; Persson, M.; Ostling, G.; Nilsson, P.M.; Melander, O.; Hedblad, B.; Engstrom, G. Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period: The malmo diet and cancer study. Atherosclerosis 2015, 239, 615–621. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Larsson, A.; Malm, J.; Grubb, A.; Hansson, L.O. Calculation of glomerular filtration rate expressed in ml/min from plasma cystatin c values in mg/l. Scand. J. Clin. Lab. Investig. 2004, 64, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.D.; Sheehan, N.A.; Scurrah, K.J.; Burton, P.R. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat. Med. 2005, 24, 2911–2935. [Google Scholar] [CrossRef] [PubMed]
- Lytsy, P.; Lind, L.; Sundstrom, J. Endothelial function and risk of hypertension and blood pressure progression: The prospective investigation of the vasculature in uppsala seniors. J. Hypertens. 2013, 31, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 aha/acc focused update of the 2014 aha/acc guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, J.; Sullivan, L.; D’Agostino, R.B.; Levy, D.; Kannel, W.B.; Vasan, R.S. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005, 45, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Arnlov, J.; Lindahl, B.; Siegbahn, A.; Sundstrom, J.; Ingelsson, E. Use of a proximity extension assay proteomics chip to discover new biomarkers for human atherosclerosis. Atherosclerosis 2015, 242, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Bjorkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Rennel Dickens, E.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-plex pea immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef]
- Benjamini Yoav, H.Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 12. [Google Scholar] [CrossRef]
- Ganna, A.; Salihovic, S.; Sundstrom, J.; Broeckling, C.D.; Hedman, A.K.; Magnusson, P.K.; Pedersen, N.L.; Larsson, A.; Siegbahn, A.; Zilmer, M.; et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014, 10, e1004801. [Google Scholar] [CrossRef]
- Ganna, A.; Lee, D.; Ingelsson, E.; Pawitan, Y. Rediscovery rate estimation for assessing the validation of significant findings in high-throughput studies. Brief. Bioinform. 2015, 16, 563–575. [Google Scholar] [CrossRef]
- The DAGitty. Available online: www.dagitty.net (accessed on 8 August 2019).
- Lieb, W.; Chen, M.H.; Teumer, A.; de Boer, R.A.; Lin, H.; Fox, E.R.; Musani, S.K.; Wilson, J.G.; Wang, T.J.; Volzke, H.; et al. Genome-wide meta-analyses of plasma renin activity and concentration reveal association with the kininogen 1 and prekallikrein genes. Circ. Cardiovasc. Genet. 2015, 8, 131–140. [Google Scholar] [CrossRef]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Konoshita, T.; Genomic Disease Outcome Consortium Study, I. Do genetic variants of the renin-angiotensin system predict blood pressure response to renin-angiotensin system-blocking drugs?: A systematic review of pharmacogenomics in the renin-angiotensin system. Curr. Hypertens. Rep. 2011, 13, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S. Sample size and power calculations in mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 2014, 43, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.; Mischak, H.; Mullen, W.; Staessen, J.A. Urinary proteome analysis in hypertensive patients with left ventricular diastolic dysfunction. Eur. Heart J. 2012, 33, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Kolialexi, A.; Mavreli, D.; Tounta, G.; Mavrou, A.; Papantoniou, N. Urine proteomic studies in preeclampsia. Proteom. Clin. Appl. 2015, 9, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Li, Y.L.; Zhang, S.J.; Yang, W.Q.; Nie, W.T.; Jiang, H.Q. Quantitative serum proteomic analysis of essential hypertension using itraq technique. BioMed Res. Int. 2017, 2017, 6761549. [Google Scholar] [CrossRef]
- Gajjala, P.R.; Jankowski, V.; Heinze, G.; Bilo, G.; Zanchetti, A.; Noels, H.; Liehn, E.; Perco, P.; Schulz, A.; Delles, C.; et al. Proteomic-biostatistic integrated approach for finding the underlying molecular determinants of hypertension in human plasma. Hypertension 2017, 70, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.A.; Jacobs, D.R., Jr.; Bahrami, H.; Peralta, C.A.; Daniels, L.B.; Lima, J.A.; Maisel, A.; Duprez, D.A. Increasing aminoterminal-pro-b-type natriuretic peptide precedes the development of arterial hypertension: The multiethnic study of atherosclerosis. J. Hypertens. 2015, 33, 966–974. [Google Scholar] [CrossRef]
- Seven, E.; Husemoen, L.L.; Ibsen, H.; Friedrich, N.; Nauck, M.; Wachtell, K.; Linneberg, A.; Jeppesen, J.L. Higher serum concentrations of n-terminal pro-b-type natriuretic peptide associate with prevalent hypertension whereas lower associate with incident hypertension. PLoS ONE 2015, 10, e0117864. [Google Scholar] [CrossRef]
- Sim, J.J.; Bhandari, S.K.; Shi, J.; Kalantar-Zadeh, K.; Rasgon, S.A.; Sealey, J.E.; Laragh, J.H. Plasma renin activity (pra) levels and antihypertensive drug use in a large healthcare system. Am. J. Hypertens. 2012, 25, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Robinson-Cohen, C.; Luque-Fernandez, M.A.; Allison, M.A.; Baudrand, R.; Ix, J.H.; Kestenbaum, B.; de Boer, I.H.; Vaidya, A. The spectrum of subclinical primary aldosteronism and incident hypertension: A cohort study. Ann. Intern. Med. 2017, 167, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Drayer, J.I.; Weber, M.A.; Laragh, J.H.; Sealey, J.E. Renin subgroups in essential hypertension. Clin. Exp. Hypertens. A 1982, 4, 1817–1834. [Google Scholar] [CrossRef]
- Musini, V.M.; Lawrence, K.A.; Fortin, P.M.; Bassett, K.; Wright, J.M. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database Syst. Rev. 2017, 4, CD007066. [Google Scholar] [CrossRef]
- Meade, T.W.; Imeson, J.D.; Gordon, D.; Peart, W.S. The epidemiology of plasma renin. Clin. Sci. (Lond.) 1983, 64, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Belmin, J.; Levy, B.I.; Michel, J.B. Changes in the renin-angiotensin-aldosterone axis in later life. Drugs Aging 1994, 5, 391–400. [Google Scholar] [CrossRef]
- Tsunoda, K.; Abe, K.; Goto, T.; Yasujima, M.; Sato, M.; Omata, K.; Seino, M.; Yoshinaga, K. Effect of age on the renin-angiotensin-aldosterone system in normal subjects: Simultaneous measurement of active and inactive renin, renin substrate, and aldosterone in plasma. J. Clin. Endocrinol. Metab. 1986, 62, 384–389. [Google Scholar] [CrossRef]
- Enroth, S.; Hallmans, G.; Grankvist, K.; Gyllensten, U. Effects of long-term storage time and original sampling month on biobank plasma protein concentrations. EBioMedicine 2016, 12, 309–314. [Google Scholar] [CrossRef]


| PIVUS† (n = 566) | ULSAM†† (n = 238) | MDC††† (n = 2659) | |
|---|---|---|---|
| Age (years) | 70.2 (0.2) | 77.6 (0.7) | 56.2 (5.7) |
| Women, n (%) | 285 (50.4) | 0 (0.0) | 1640 (61.7) |
| Smoker, n (%) | 55 (9.7) | 16 (6.7) | 498 (18.7) |
| Systolic blood pressure at baseline (mmHg) | 144.9 (21.1) | 146.8 (19.3) | 136.8 (17.2) |
| Diastolic blood pressure at baseline (mmHg) | 77.0 (9.9) | 78.9 (9.3) | 85.1 (8.6) |
| Systolic blood pressure at follow-up (mmHg) | 148.8 (19.8) | 145.4 (16.5) | 147.4 (20.1) |
| Diastolic blood pressure at follow-up (mmHg) | 76.5 (9.5) | 81.0 (9.2) | 85.2 (10.5) |
| Body mass index (kg/m2) | 26.5 (4.0) | 25.6 (3.0) | 25.2 (3.5) |
| Waist circumference (cm) | 89.5 (11.1) | 93.0 (9.6) | 81.5 (11.9) |
| Diabetes mellitus, n (%) | 35 (6.2) | 16 (6.7) | 119 (4.5) |
| Total cholesterol (mmol/L) | 5.5 (1.0) | 5.5 (1.0) | 6.1 (1.1) |
| Low-density lipoprotein cholesterol (mmol/L) | 3.4 (0.9) | 3.6 (0.8) | 4.1 (1.0) |
| High-density lipoprotein cholesterol (mmol/L) | 1.6 (0.4) | 1.4 (0.3) | 1.4 (0.4) |
| Triglycerides (mmol/L) | 1.2 (0.6) | 1.3 (0.7) | 1.2 (0.6) |
| Fasting glucose (mmol/L) | 5.8 (1.3) | 5.7 (1.4) | 5.5 (0.9) |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 71.6 (14.9) | 78.4 (14.5) | 83.9 (14.1) |
| Statin treatment, n (%) | 68 (12.0) | 27 (11.3) | 31 (1.2) |
| Baseline examination starting year | 2001 | 1998 | 1991 |
| Anti-hypertensive treatment during follow-up, n (%) | 163 (28.8) | 65 (27.3) | 1354 (50.9) |
| Length of follow-up (years) | 5.1 (0.1) | 4.1 (0.6) | 16.7 (1.5) |
| OR (95% CI) | p-Value | |
|---|---|---|
| Discovery sample (PIVUS and ULSAM) | 1.33 (1.14 to1.56) | <0.001 |
| Replication sample (MDC) | 1.07 (0.97 to 1.19) | 0.199 |
| Meta-analysis of all three cohorts | 1.08 (1.01 to 1.15) | 0.030 |
| SBP | DBP | |||
|---|---|---|---|---|
| Linear Mixed Regression | Linear Mixed Regression | |||
| β-Coefficient | 95% CI | β-Coefficient | 95% CI | |
| Discovery sample (PIVUS and ULSAM) | 1.94 | 0.65 to 3.23 | 0.84 | 0.11 to 1.58 |
| Replication sample (MDC) | −0.52 | −1.62 to 0.58 | 0.48 | −0.14 to 1.10 |
| Meta-analysis of all three cohorts | 0.69 | 0.36 to 1.03 | 0.43 | −0.02 to 0.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Fall, T.; Hammar, U.; Gustafsson, S.; Ingelsson, E.; Ärnlöv, J.; Lind, L.; Engström, G.; Sundström, J. Proteomic Analysis of Longitudinal Changes in Blood Pressure. J. Clin. Med. 2019, 8, 1585. https://doi.org/10.3390/jcm8101585
Lin Y-T, Fall T, Hammar U, Gustafsson S, Ingelsson E, Ärnlöv J, Lind L, Engström G, Sundström J. Proteomic Analysis of Longitudinal Changes in Blood Pressure. Journal of Clinical Medicine. 2019; 8(10):1585. https://doi.org/10.3390/jcm8101585
Chicago/Turabian StyleLin, Yi-Ting, Tove Fall, Ulf Hammar, Stefan Gustafsson, Erik Ingelsson, Johan Ärnlöv, Lars Lind, Gunnar Engström, and Johan Sundström. 2019. "Proteomic Analysis of Longitudinal Changes in Blood Pressure" Journal of Clinical Medicine 8, no. 10: 1585. https://doi.org/10.3390/jcm8101585