Decreasing Trends of Secondary Primary Colorectal Cancer among Women with Uterine Cancer: A Population-Based Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Eligibility
2.2. Clinical Information
2.3. Study Definition
2.4. Study Aim
2.5. Statistical Consideration
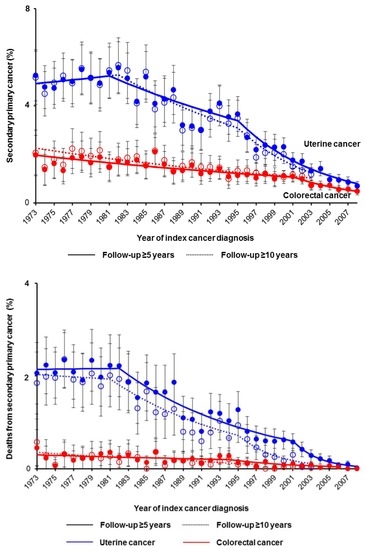
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Wright, J.D.; Barrena Medel, N.I.; Sehouli, J.; Fujiwara, K.; Herzog, T.J. Contemporary management of endometrial cancer. Lancet 2012, 379, 1352–1360. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2015, 387, 1094–1108. [Google Scholar] [CrossRef]
- Lu, K.H.; Dinh, M.; Kohlmann, W.; Watson, P.; Green, J.; Syngal, S.; Bandipalliam, P.; Chen, L.M.; Allen, B.; Conrad, P.; et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet. Gynecol. 2005, 105, 569–574. [Google Scholar] [CrossRef]
- Weinberg, D.S.; Newschaffer, C.J.; Topham, A. Risk for colorectal cancer after gynecologic cancer. Ann. Intern. Med. 1999, 131, 189–193. [Google Scholar] [CrossRef]
- Brown, A.P.; Neeley, E.S.; Werner, T.; Soisson, A.P.; Burt, R.W.; Gaffney, D.K. A population-based study of subsequent primary malignancies after endometrial cancer: Genetic, environmental, and treatment-related associations. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Chan, A.T.; Ogino, S. Anatomic subsite of primary colorectal cancer and subsequent risk and distribution of second cancers. Cancer 2013, 119, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Won, Y.J.; Lim, J.; Seo, S.S.; Kang, S.; Yoo, C.W.; Kim, J.Y.; Oh, J.H.; Bristow, R.E.; Park, S.Y. Second primary colorectal cancer among endometrial cancer survivor: Shared etiology and treatment sequelae. J. Cancer Res. Clin. Oncol. 2018, 144, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Nugent, Z.; Demers, A.; Czaykowski, P.M.; Mahmud, S.M. Risk of colorectal cancer after diagnosis of endometrial cancer: A population-based study. J. Clin. Oncol. 2013, 31, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Onsrud, M.; Cvancarova, M.; Hellebust, T.P.; Trope, C.G.; Kristensen, G.B.; Lindemann, K. Long-term outcomes after pelvic radiation for early-stage endometrial cancer. J. Clin. Oncol. 2013, 31, 3951–3956. [Google Scholar] [CrossRef]
- Rombouts, A.J.M.; Hugen, N.; van Beek, J.J.P.; Poortmans, P.M.P.; de Wilt, J.H.W.; Nagtegaal, I.D. Does pelvic radiation increase rectal cancer incidence?—A systematic review and meta-analysis. Cancer Treat Rev. 2018, 68, 136–144. [Google Scholar] [CrossRef]
- Moreira, L.; Balaguer, F.; Lindor, N.; de la Chapelle, A.; Hampel, H.; Aaltonen, L.A.; Hopper, J.L.; Le Marchand, L.; Gallinger, S.; Newcomb, P.A.; et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA 2012, 308, 1555–1565. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin No. 147: Lynch syndrome. Obstet. Gynecol. 2014, 124, 1042–1054.
- An Aging World: 2015. International Population Reports. United States Census Bureau. Available online: https://www.census.gov/content/dam/Census/library/publications/2016/demo/p95-16-1.pdf (accessed on 14 April 2019).
- Health, United States, 2016. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/nchs/data/hus/hus16.pdf#053 (accessed on 14 April 2019).
- Colorectal Cancer Screening, Incidence, and Mortality—United States, 2002–2010. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6026a4.htm (accessed on 14 April 2019).
- NIH State-of-the-Science Conference: Enhancing Use and Quality of Colorectal Cancer Screening. NIH Consensus Development Program Office of Disease Prevention. Available online: https://consensus.nih.gov/2010/colorectalstatement.htm (accessed on 14 April 2019).
- The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Available online: https://seer.cancer.gov/ (accessed on 15 April 2019).
- National Cancer Registrars Association. Available online: http://www.ncra-usa.org/i4a/pages/index.cfm?pageid=1%3e (accessed on 15 April 2019).
- Matsuo, K.; Machida, H.; Blake, E.A.; Holman, L.L.; Rimel, B.J.; Roman, L.D.; Wright, J.D. Trends and outcomes of women with synchronous endometrial and ovarian cancer. Oncotarget 2018, 9, 28757–28771. [Google Scholar] [CrossRef]
- Matsuo, K.; Machida, H.; Stone, R.L.; Soliman, P.T.; Thaker, P.H.; Roman, L.D.; Wright, J.D. Risk of Subsequent Ovarian Cancer After Ovarian Conservation in Young Women with Stage I Endometrioid Endometrial Cancer. Obstet. Gynecol. 2017, 130, 403–410. [Google Scholar] [CrossRef]
- AIRTUM Working Group. Italian cancer figures, report 2013: Multiple tumours. Epidemiol. Prev. 2013, 37, 1–152. [Google Scholar]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Matsuo, K.; Machida, H.; Shoupe, D.; Melamed, A.; Muderspach, L.I.; Roman, L.D.; Wright, J.D. Ovarian Conservation and Overall Survival in Young Women with Early-Stage Low-Grade Endometrial Cancer. Obstet. Gynecol. 2016, 128, 761–770. [Google Scholar] [CrossRef]
- National Death Index. Available online: https://www.cdc.gov/nchs/ndi/ (accessed on 14 April 2019).
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Series B Stat. Methodol. 1972, 34, 187–220. [Google Scholar] [CrossRef]
- National Cancer Institute Joinpoint Trend Analysis Software. Available online: http://surveillance.cancer.gov/joinpoint (accessed on 14 April 2019).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Cancer stat facts. National Cancer Institute Surveillance Epidemiology End Result Program. Available online: https://seer.cancer.gov/statfacts/ (accessed on 14 April 2019).
- Rosso, S.; De Angelis, R.; Ciccolallo, L.; Carrani, E.; Soerjomataram, I.; Grande, E.; Zigon, G.; Brenner, H. Multiple tumours in survival estimates. Eur. J. Cancer 2009, 45, 1080–1094. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Colorectal cancer screening. National Cncer Institute Cancer Trends Progress Reports. Available online: https://progressreport.cancer.gov/detection/colorectal_cancer (accessed on 14 April 2019).
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W., Jr.; Garcia, F.A.R.; Gillman, M.W.; Harper, D.M.; Kemper, A.R.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 315, 2564–2575. [Google Scholar]
- Cancer stat facts: Colorectal cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 14 April 2019).
- Cohen, S.A. Current Lynch syndrome tumor screening practices: A survey of genetic counselors. J. Genet. Couns. 2013, 23, 38–47. [Google Scholar] [CrossRef]
- Lindor, N.M.; Petersen, G.M.; Hadley, D.W.; Kinney, A.Y.; Miesfeldt, S.; Lu, K.H.; Lynch, P.; Burke, W.; Press, N. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: A systematic review. JAMA 2006, 296, 1507–1517. [Google Scholar] [CrossRef]
- Yurgelun, M.B.; Mercado, R.; Rosenblatt, M.; Dandapani, M.; Kohlmann, W.; Conrad, P.; Blanco, A.; Shannon, K.M.; Chung, D.C.; Terdiman, J.; et al. Impact of genetic testing on endometrial cancer risk-reducing practices in women at risk for Lynch syndrome. Gynecol. Oncol. 2012, 127, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, K.; Machida, H.; Ragab, O.M.; Takiuchi, T.; Pham, H.Q.; Roman, L.D. Extent of pelvic lymphadenectomy and use of adjuvant vaginal brachytherapy for early-stage endometrial cancer. Gynecol. Oncol. 2017, 144, 515–523. [Google Scholar] [CrossRef]
- Curtis, R.E.; Freedman, D.M.; Ron, E.; Ries, L.A.G.; Hacker, D.G.; Edwards, B.K.; Tucker, M.A.; Fraumeni, J.F. New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973–2000; National Cancer Institute (NCI): Bethesda, MD, USA, 2006; pp. 9–14.




| Characteristic | No CRC | Antecedent-CRC | Synchronous-CRC | Postcedent-CRC | p-Value |
|---|---|---|---|---|---|
| Number | n = 239,410 | n = 2103 | n = 615 | n = 4093 | |
| Age | 62.9 (± 12.6) | 70.5 (± 12.4) | 68.5 (± 12.4) | 63.1 (± 11.5) | <0.001 |
| Year | <0.001 | ||||
| Before 1980 | 20,564 (8.6%) | 59 (2.8%) | 51 (8.3%) | 883 (21.6%) | |
| 1980–1989 | 27,218 (11.4%) | 238 (11.3%) | 80 (13.0%) | 978 (23.9%) | |
| 1990–1999 | 39,864 (16.7%) | 448 (21.3%) | 121 (19.7%) | 987 (24.1%) | |
| 2000–2009 | 100,853 (42.1%) | 814 (38.7%) | 252 (41.0%) | 1125 (27.5%) | |
| 2010 or later | 50,911 (21.3%) | 544 (25.9%) | 111 (18.0%) | 120 (2.9%) | |
| Race/ethnicity | <0.001 | ||||
| White | 184,497 (77.1%) | 1661 (79.0%) | 499 (81.1%) | 3445 (84.2%) | |
| Black | 18,661 (7.8%) | 153 (7.3%) | 58 (9.4%) | 238 (5.8%) | |
| Hispanic | 18,993 (7.9%) | 137 (6.5%) | 32 (5.2%) | 170 (4.2%) | |
| Asian | 12,611 (5.3%) | 121 (5.8%) | 21 (3.4%) | 189 (4.6%) | |
| Others * | 4648 (1.9%) | 31 (1.5%) | 5 (0.8%) | 51 (1.2%) | |
| Area | <0.001 | ||||
| West | 123,311 (51.5%) | 1021 (48.5%) | 260 (42.3%) | 1966 (48.0%) | |
| Central | 55,898 (23.3%) | 584 (27.8%) | 168 (27.3%) | 1195 (29.2%) | |
| East | 60,201 (25.1%) | 498 (23.7%) | 187 (30.4%) | 932 (22.8%) | |
| Marital status | <0.001 | ||||
| Single | 35,108 (14.7%) | 205 (9.7%) | 87 (14.1%) | 475 (11.6%) | |
| Married | 123,912 (51.8%) | 945 (44.9%) | 293 (47.6%) | 2290 (55.9%) | |
| Others * | 80,390 (33.6%) | 953 (45.3%) | 235 (38.2%) | 1328 (32.4%) | |
| Histology types | <0.001 | ||||
| Endometrioid | 172,784 (72.2%) | 1416 (67.3%) | 416 (67.6%) | 3250 (79.4%) | |
| Serous | 13,975 (5.8%) | 173 (8.2%) | 55 (8.9%) | 221 (5.4%) | |
| Clear cell | 2938 (1.2%) | 45 (2.1%) | 13 (2.1%) | 41 (1.0%) | |
| Carcinosarcoma | 10,664 (4.5%) | 159 (7.6%) | 28 (4.6%) | 84 (2.1%) | |
| Sarcoma | 10,376 (4.3%) | 44 (2.1%) | 20 (3.3%) | 69 (1.7%) | |
| Mixed | 7318 (3.1%) | 95 (4.5%) | 17 (2.8%) | 45 (1.1%) | |
| Others | 21,357 (8.9%) | 171 (8.1%) | 66 (10.7%) | 383 (9.4%) | |
| Stage | <0.001 | ||||
| I | 150,210 (62.7%) | 1263 (60.1%) | 326 (53.0%) | 2525 (61.7%) | |
| II | 10,034 (4.2%) | 119 (5.7%) | 33 (5.4%) | 172 (4.2%) | |
| III | 20,446 (8.5%) | 221 (10.5%) | 64 (10.4%) | 219 (5.4%) | |
| IV | 18,163 (7.6%) | 165 (7.8%) | 65 (10.6%) | 111 (2.7%) | |
| Unknown | 40,557 (16.9%) | 335 (15.9%) | 127 (20.7%) | 1066 (26.0%) | |
| Grade | <0.001 | ||||
| 1 | 83,271 (34.8%) | 538 (25.6%) | 174 (28.3%) | 1514 (37.0%) | |
| 2 | 62,105 (25.9%) | 559 (26.6%) | 163 (26.5%) | 1307 (31.9%) | |
| 3 | 51,833 (21.7%) | 609 (29.0%) | 149 (24.2%) | 666 (16.3%) | |
| Unknown | 42,201 (17.6%) | 397 (18.9%) | 129 (21.0%) | 606 (14.8%) | |
| Tumor size | <0.001 | ||||
| ≤2 cm | 21,820 (9.1%) | 205 (9.7%) | 47 (7.6%) | 284 (6.9%) | |
| >2 cm | 81,395 (34.0%) | 745 (35.4%) | 185 (30.1%) | 893 (21.8%) | |
| Unknown | 136,194 (56.9%) | 1153 (54.8%) | 383 (62.3%) | 2916 (71.2%) | |
| Pelvic lymph node † | <0.001 | ||||
| Not involved | 167,161 (91.6%) | 1444 (91.7%) | 402 (92.0%) | 2439 (95.6%) | |
| Involved | 15,236 (8.4%) | 131 (8.3%) | 35 (8.0%) | 112 (4.4%) | |
| Lymph node ratio (%) † | 20.0 (9.1-50.0) | 33.3 (12.5-57.1) | 31.3 (17.0-100) | 18.6 (10.0-38.2) | 0.004 |
| Hysterectomy | <0.001 | ||||
| No | 19,763 (8.3%) | 306 (14.6%) | 64 (10.4%) | 102 (2.5%) | |
| Yes | 187,201 (78.2%) | 1657 (78.8%) | 451 (73.3%) | 2744 (67.0%) | |
| Unknown | 32,446 (13.6%) | 140 (6.7%) | 100 (16.3%) | 1247 (30.5%) | |
| Radiotherapy | <0.001 | ||||
| None | 165,520 (69.1%) | 1534 (72.9%) | 470 (76.4%) | 2486 (60.7%) | |
| External beam | 48,940 (20.4%) | 358 (17.0%) | 108 (17.6%) | 1117 (27.3%) | |
| Implants | 13,082 (5.5%) | 130 (6.2%) | 19 (3.1%) | 138 (3.4%) | |
| Both | 6861 (2.9%) | 32 (1.5%) | 6 (1.0%) | 290 (7.1%) | |
| Unknown | 5007 (2.1%) | 49 (2.3%) | 12 (2.0%) | 62 (1.5%) |
| Cause-Specific Survival | Overall Survival | ||||
|---|---|---|---|---|---|
| Characteristic | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| All histology | Secondary colorectal cancer | ||||
| No | 1 | 1 | |||
| Yes (any timing) | 0.69 (0.67–0.72) | <0.001 | 1.01 (0.99–1.03) | 0.08 | |
| Secondary colorectal cancer | |||||
| No | 1 | 1 | |||
| Antecedent | 0.82 (0.74–0.91) | <0.001 | 1.26 (1.19–1.33) | <0.001 | |
| Synchronous | 0.77 (0.63–0.93) | 0.008 | 1.55 (1.41–1.71) | <0.001 | |
| Postcedent | 0.26 (0.23–0.30) | <0.001 | 0.91 (0.88–0.94) | <0.001 | |
| Endometrial | Secondary colorectal cancer | ||||
| No | 1 | 1 | |||
| Yes (any timing) | 0.70 (0.67–0.73) | <0.001 | 1.02 (1.01–1.04) | 0.005 | |
| Secondary colorectal cancer | |||||
| No | 1 | 1 | |||
| Antecedent | 0.82 (0.74–0.92) | 0.001 | 1.26 (1.19–1.33) | <0.001 | |
| Synchronous | 0.81 (0.66–0.99) | 0.046 | 1.67 (1.5–1.85) | <0.001 | |
| Postcedent | 0.28 (0.24–0.32) | <0.001 | 0.93 (0.90–0.97) | <0.001 | |
| Endometrioid | Secondary colorectal cancer | ||||
| No | 1 | 1 | |||
| Yes (any timing) | 0.68 (0.63–0.70) | <0.001 | 1.04 (1.02–1.06) | <0.001 | |
| Secondary colorectal cancer | |||||
| No | 1 | 1 | |||
| Antecedent | 0.78 (0.67–0.91) | 0.002 | 1.32 (1.23–1.42) | <0.001 | |
| Synchronous | 0.65 (0.50–0.86) | 0.002 | 1.55 (1.37–1.75) | <0.001 | |
| Postcedent | 0.29 (0.25–0.34) | <0.001 | 0.99 (0.95–1.03) | 0.55 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, K.; Mandelbaum, R.S.; Machida, H.; Yoshihara, K.; Muggia, F.M.; Roman, L.D.; Wright, J.D. Decreasing Trends of Secondary Primary Colorectal Cancer among Women with Uterine Cancer: A Population-Based Analysis. J. Clin. Med. 2019, 8, 714. https://doi.org/10.3390/jcm8050714
Matsuo K, Mandelbaum RS, Machida H, Yoshihara K, Muggia FM, Roman LD, Wright JD. Decreasing Trends of Secondary Primary Colorectal Cancer among Women with Uterine Cancer: A Population-Based Analysis. Journal of Clinical Medicine. 2019; 8(5):714. https://doi.org/10.3390/jcm8050714
Chicago/Turabian StyleMatsuo, Koji, Rachel S. Mandelbaum, Hiroko Machida, Kosuke Yoshihara, Franco M. Muggia, Lynda D. Roman, and Jason D. Wright. 2019. "Decreasing Trends of Secondary Primary Colorectal Cancer among Women with Uterine Cancer: A Population-Based Analysis" Journal of Clinical Medicine 8, no. 5: 714. https://doi.org/10.3390/jcm8050714