Hepatitis B Virus Reactivation with Immunosuppression: A Hidden Threat?
Abstract
:1. Introduction
2. Sources and Selection Criteria
3. Hepatitis B Reactivation: An Overview
3.1. Definitions
3.2. Natural History and Risk Factors for HBV Reactivation
4. Immunosuppressive Agents
4.1. Risk Stratification Overview
4.2. Oncology
4.2.1. Anti-CD20/B-Cell-Depleting Agents
4.2.2. Hematopoietic Stem Cell Transplant (HSCT)
4.2.3. Other Immunotherapy: Checkpoint Inhibitors, Tyrosine Kinase Inhibitors, and mTOR Inhibitors (Everolimus)
4.2.4. Chemotherapy: Alkylating Agents (Cyclophosphamide and Etoposide) and Anthracyclines
4.2.5. CAR T-Cell Immunotherapy
4.3. Other Immunosuppressive Agents
4.3.1. Glucocorticoids
4.3.2. Antiproliferative Agents (Methotrexate)
4.3.3. Calcineurin Inhibitors
4.3.4. Cytokine Inhibitors (Tocilizumab, Ustekinumab, Secukinumab, and Siltuximab)
4.3.5. Anti-TNF Agents (Infliximab, Adalimumab, Certolizumab, and Etanercept)
4.3.6. Janus Kinase (JAK) Inhibitors (Baricitinib and Tofacitinib)
4.3.7. T-Cell-Depleting Agents (Abatacept and Alemtuzumab)
4.4. Other Immunosuppressed States
HIV Co-Infection
5. Discussion
5.1. An Approach to Immunosuppression in Patients with HBV
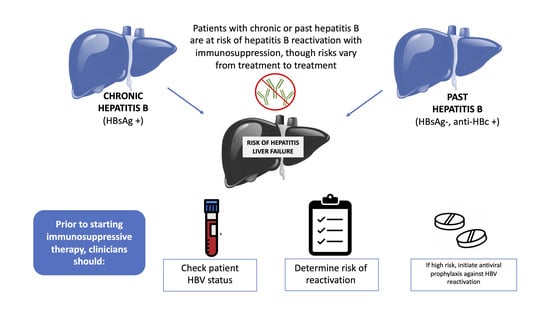
5.1.1. Screening
5.1.2. Antiviral Prophylaxis: Choice of Agent and Duration of Therapy
5.1.3. Prophylaxis and Suggested Monitoring
5.1.4. Treatment of HBV Reactivation
5.2. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Revill, P.A.; Chisari, F.V.; Block, J.M.; Dandri, M.; Gehring, A.J.; Guo, H.; Hu, J.; Kramvis, A.; Lampertico, P.; Janssen, H.L.A.; et al. A Global Scientific Strategy to Cure Hepatitis B. Lancet Gastroenterol. Hepatol. 2019, 4, 545–558. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030; World Health Organization: Geneva, Switzerland, 2022.
- Waheed, Y. Progress on Global Hepatitis Elimination Targets. World J. Gastroenterol. 2021, 27, 8199–8200. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory Collaborators. Global Prevalence, Cascade of Care, and Prophylaxis Coverage of Hepatitis B in 2022: A Modelling Study. Lancet Gastroenterol. Hepatol. 2023, 8, 879–907. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.; Seaman, C.; Wallace, J.; Xiao, Y.; Scott, N.; Davies, J.; de Santis, T.; Adda, D.; El-Sayed, M.; Feld, J.J.; et al. Pathway to Global Elimination of Hepatitis B: HBV Cure Is Just the First Step. Hepatology 2023, 78, 976–990. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Lim, S.-G.; Plesniak, R.; Tsuji, K.; Janssen, H.L.A.; Pojoga, C.; Gadano, A.; Popescu, C.P.; Stepanova, T.; Asselah, T.; et al. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection. N. Engl. J. Med. 2022, 387, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- GlaxoSmithKline. Study of Bepirovirsen in Nucleos(t)Ide Analogue-Treated Participants with Chronic Hepatitis B (B-Well 1) (B-Well 1). Available online: https://clinicaltrials.gov/study/NCT05630807 (accessed on 22 December 2023).
- GlaxoSmithKline. Study of Bepirovirsen in Nucleos(t)Ide Analogue-Treated Participants with Chronic Hepatitis B (B-Well 2) (B-Well 2). Available online: https://clinicaltrials.gov/study/NCT05630820 (accessed on 22 December 2023).
- Shi, Y.; Zheng, M. Hepatitis B Virus Persistence and Reactivation. BMJ 2020, 1, 370. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.; Hwang, J.P.; Jonas, M.M.; Brown, R.S.; Bzowej, N.H.; Wong, J.B. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Davis, G.L.; Hoofnagle, J.H.; Waggoner, J.G. Spontaneous Reactivation of Chronic Hepatitis B Virus Infection. Gastroenterology 1984, 86, 230–235. [Google Scholar] [CrossRef]
- Kamitsukasa, H.; Iri, M.; Tanaka, A.; Nagashima, S.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Spontaneous Reactivation of Hepatitis B Virus (HBV) Infection in Patients with Resolved or Occult HBV Infection. J. Med. Virol. 2015, 87, 589–600. [Google Scholar] [CrossRef]
- Rehermann, B. Pathogenesis of Chronic Viral Hepatitis: Differential Roles of T Cells and NK Cells. Nat. Med. 2013, 19, 859–868. [Google Scholar] [CrossRef]
- Smalls, D.J.; Kiger, R.E.; Norris, L.B.; Bennett, C.L.; Love, B.L. Hepatitis B Virus Reactivation: Risk Factors and Current Management Strategies. Pharmacotherapy 2019, 39, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Wands, J.R.; Chura, C.M.; Roll, F.J.; Maddrey, W.C. Serial Studies of Hepatitis-Associated Antigen and Antibody in Patients Receiving Antitumor Chemotherapy for Myeloproliferative and Lymphoproliferative Disorders. Gastroenterology 1975, 68, 105–112. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Dusheiko, G.M.; Schafer, D.F.; Jones, E.A.; Micetich, K.C.; Young, R.C.; Costa, J. Reactivation of Chronic Hepatitis B Virus Infection by Cancer Chemotherapy. Ann. Intern. Med. 1982, 96, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Dickstein, A.; Saxena, A.; Terrin, N.; Viveiros, K.; Balk, E.M.; Wong, J.B. Role of Surface Antibody in Hepatitis B Reactivation in Patients with Resolved Infection and Hematologic Malignancy: A Meta-Analysis. Hepatology 2017, 66, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Lekakis, V.; Voulgaris, T.; Lampertico, P.; Berg, T.; Chan, H.L.Y.; Kao, J.-H.; Terrault, N.; Lok, A.S.; Reddy, K.R. Hepatitis B Virus Reactivation Associated with New Classes of Immunosuppressants and Immunomodulators: A Systematic Review, Meta-Analysis, and Expert Opinion. J. Hepatol. 2022, 77, 1670–1689. [Google Scholar] [CrossRef]
- Myint, A.; Tong, M.J.; Beaven, S.W. Reactivation of Hepatitis B Virus: A Review of Clinical Guidelines. Clin. Liver Dis. 2020, 15, 162–167. [Google Scholar] [CrossRef]
- Loomba, R.; Liang, T.J. Hepatitis B Reactivation Associated with Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology 2017, 152, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.; Yu, M.-L.; Wong, G.; Thompson, A.; Ghazinian, H.; Hou, J.-L.; Piratvisuth, T.; Jia, J.-D.; Mizokami, M.; Cheng, G.; et al. APASL Clinical Practice Guideline on Hepatitis B Reactivation Related to the Use of Immunosuppressive Therapy. Hepatol. Int. 2021, 15, 1031–1048. [Google Scholar] [CrossRef]
- Yeo, W.; Johnson, P.J. Diagnosis, Prevention and Management of Hepatitis B Virus Reactivation during Anticancer Therapy. Hepatology 2006, 43, 209–220. [Google Scholar] [CrossRef]
- Lok, A.S.F.; McMahon, B.J. Chronic Hepatitis B: Update 2009. Hepatology 2009, 50, 661–662. [Google Scholar] [CrossRef]
- Velkov, S.; Ott, J.J.; Protzer, U.; Michler, T. The Global Hepatitis B Virus Genotype Distribution Approximated from Available Genotyping Data. Genes 2018, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.P.; Feld, J.J.; Hammond, S.P.; Wang, S.H.; Alston-Johnson, D.E.; Cryer, D.R.; Hershman, D.L.; Loehrer, A.P.; Sabichi, A.L.; Symington, B.E.; et al. Hepatitis B Virus Screening and Management for Patients with Cancer Prior to Therapy: ASCO Provisional Clinical Opinion Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3698–3715. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Jovanovic, B.D.; Su, Y.-C.; Raisch, D.W.; Ganger, D.; Belknap, S.M.; Dai, M.-S.; Chiu, B.-C.C.; Fintel, B.; Cheng, Y.; et al. Rituximab-Associated Hepatitis B Virus (HBV) Reactivation in Lymphoproliferative Diseases: Meta-Analysis and Examination of FDA Safety Reports. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1170–1180. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Yamamoto, Y.; Ito, S.; Ohigashi, H.; Shiratori, S.; Naruse, H.; Teshima, T. Hepatitis B Virus Reactivation with a Rituximab-Containing Regimen. World J. Hepatol. 2015, 7, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Rowley, A.; Wesley, R.; Liang, T.J.; Hoofnagle, J.H.; Pucino, F.; Csako, G. Systematic Review: The Effect of Preventive Lamivudine on Hepatitis B Reactivation during Chemotherapy. Ann. Intern. Med. 2008, 148, 519–528. [Google Scholar] [CrossRef]
- Hoofnagle, J.H. Reactivation of Hepatitis B. Hepatology 2009, 49, S156–S165. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Reddy, K.R.; Beavers, K.L.; Hammond, S.P.; Lim, J.K.; Falck-Ytter, Y.T.; American Gastroenterological Association Institute. American Gastroenterological Association Institute Guideline on the Prevention and Treatment of Hepatitis B Virus Reactivation during Immunosuppressive Drug Therapy. Gastroenterology 2015, 148, 215–219; quiz e16–e17. [Google Scholar] [CrossRef]
- Nakamoto, S.; Kanda, T.; Nakaseko, C.; Sakaida, E.; Ohwada, C.; Takeuchi, M.; Takeda, Y.; Mimura, N.; Iseki, T.; Wu, S.; et al. Reactivation of Hepatitis B Virus in Hematopoietic Stem Cell Transplant Recipients in Japan: Efficacy of Nucleos(t)Ide Analogues for Prevention and Treatment. Int. J. Mol. Sci. 2014, 15, 21455–21467. [Google Scholar] [CrossRef]
- Lau, G.K.K.; He, M.-L.; Fong, D.Y.T.; Bartholomeusz, A.; Au, W.-Y.; Lie, A.K.W.; Locarnini, S.; Liang, R. Preemptive Use of Lamivudine Reduces Hepatitis B Exacerbation after Allogeneic Hematopoietic Cell Transplantation. Hepatology 2002, 36, 702–709. [Google Scholar] [CrossRef]
- Mikulska, M.; Nicolini, L.; Signori, A.; Rivoli, G.; Del Bono, V.; Raiola, A.M.; Di Grazia, C.; Dominietto, A.; Varaldo, R.; Ghiso, A.; et al. Hepatitis B Reactivation in HBsAg-Negative/HBcAb-Positive Allogeneic Haematopoietic Stem Cell Transplant Recipients: Risk Factors and Outcome. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, O694–O701. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Yamagishi, Y.; Ebinuma, H.; Nakamoto, N.; Katahira, M.; Sasaki, A.; Sakamoto, M.; Suzuki, H.; Kanai, T.; Hibi, T. Progressive Liver Failure Induced by Everolimus for Renal Cell Carcinoma in a 58-Year-Old Male Hepatitis B Virus Carrier. Clin. J. Gastroenterol. 2013, 6, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Shiah, H.-S.; Chen, C.-Y.; Dai, C.-Y.; Hsiao, C.-F.; Lin, Y.-J.; Su, W.-C.; Chang, J.-Y.; Whang-Peng, J.; Lin, P.-W.; Huang, J.-D.; et al. Randomised Clinical Trial: Comparison of Two Everolimus Dosing Schedules in Patients with Advanced Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2013, 37, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Karaca, M.; Tural, D.; Akar, E.; Çil, İ.; Bayrak, S.; Ozet, G.; Yucel, O.K.; Hocaoglu, E.; Ozet, A. Hepatitis B Reactivation Rate Is Higher Undergoing Some Cytotoxic Chemotherapy in Patients with Solid Tumors: A Large Retrospective Study. Chemotherapy 2018, 63, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Ahn, J.H.; Kim, S.-B.; Im, Y.-S.; Lee, S.I.; Ahn, S.-H.; Son, B.H.; Gong, G.; Kim, H.-H.; Kim, W.K. Hepatitis B Reactivation during Adjuvant Anthracycline-Based Chemotherapy in Patients with Breast Cancer: A Single Institution’s Experience. Korean J. Intern. Med. 2007, 22, 237–243. [Google Scholar] [CrossRef]
- Zhong, Z.; Liao, W.; Dai, L.; Feng, X.; Su, G.; Gao, Y.; Wu, Q.; Yang, P. Average Corticosteroid Dose and Risk for HBV Reactivation and Hepatitis Flare in Patients with Resolved Hepatitis B Infection. Ann. Rheum. Dis. 2022, 81, 584–591. [Google Scholar] [CrossRef]
- Rodríguez-Tajes, S.; Miralpeix, A.; Costa, J.; López-Suñé, E.; Laguno, M.; Pocurull, A.; Lens, S.; Mariño, Z.; Forns, X. Low Risk of Hepatitis B Reactivation in Patients with Severe COVID-19 Who Receive Immunosuppressive Therapy. J. Viral Hepat. 2021, 28, 89–94. [Google Scholar] [CrossRef]
- Akiyama, S.; Cotter, T.G.; Sakuraba, A. Risk of Hepatitis B Virus Reactivation in Patients with Autoimmune Diseases Undergoing Non-Tumor Necrosis Factor-Targeted Biologics. World J. Gastroenterol. 2021, 27, 2312–2324. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Huang, W.-N.; Wu, Y.-D.; Lin, C.-T.; Chen, Y.-H.; Chen, D.-Y.; Hsieh, T.-Y. Reactivation of Hepatitis B Virus Infection in Patients with Rheumatoid Arthritis Receiving Tofacitinib: A Real-World Study. Ann. Rheum. Dis. 2018, 77, 780–782. [Google Scholar] [CrossRef]
- Harigai, M.; Winthrop, K.; Takeuchi, T.; Hsieh, T.-Y.; Chen, Y.-M.; Smolen, J.S.; Burmester, G.; Walls, C.; Wu, W.-S.; Dickson, C.; et al. Evaluation of Hepatitis B Virus in Clinical Trials of Baricitinib in Rheumatoid Arthritis. RMD Open 2020, 6, e001095. [Google Scholar] [CrossRef]
- Conners, E.E.; Panagiotakopoulos, L.; Hofmeister, M.G.; Spradling, P.R.; Hagan, L.M.; Harris, A.M.; Rogers-Brown, J.S.; Wester, C.; Nelson, N.P.; Contributors; et al. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations—United States, 2023. MMWR Recomm. Rep. 2023, 72, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Poola, S.; Kratzer, M.; Sewell, K.; Tillmann, H.L. Size Matters! Anti-HBs Titer and HBV Reactivation During Anti-TNF Therapy. Dig. Dis. Sci. 2023, 68, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- Suda, G.; Baba, M.; Yamamoto, Y.; Sho, T.; Ogawa, K.; Kimura, M.; Hosoda, S.; Yoshida, S.; Kubo, A.; Fu, Q.; et al. Prophylactic Tenofovir Alafenamide for Hepatitis B Virus Reactivation and Reactivation-Related Hepatitis. J. Med. Virol. 2023, 95, e28452. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Tsou, H.-H.; Lin, S.-J.; Wang, M.-C.; Yao, M.; Hwang, W.-L.; Kao, W.-Y.; Chiu, C.-F.; Lin, S.-F.; Lin, J.; et al. Chemotherapy-Induced Hepatitis B Reactivation in Lymphoma Patients with Resolved HBV Infection: A Prospective Study. Hepatology 2014, 59, 2092–2100. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Lee, W.M. Acute Liver Failure. Lancet Lond. Engl. 2019, 394, 869–881. [Google Scholar] [CrossRef]
- Hakami, T. Acute Liver Failure Due to Hepatitis B Virus Reactivation Induced by Doxorubicin and Cyclophosphamide Chemotherapy for Adjuvant Treatment of Breast Cancer: A Case Report. Clin. Case Rep. 2022, 10, e05894. [Google Scholar] [CrossRef]
- Hegazy, Y.; Reif, M.; McGuire, B.M.; Cannon, R.; Orandi, B.; Shoreibah, M. Liver Transplantation in Hepatitis B Reactivation in a Patient with Active HIV Viremia. ACG Case Rep. J. 2022, 9, e00943. [Google Scholar] [CrossRef]



| Risk of Reactivation | HBsAg Positive (Chronic HBV) | HbsAg Negative, anti-HBc Positive (Past HBV) |
|---|---|---|
| High (>10%) | Anti-CD20 * Hematopoietic stem cell transplant Immune checkpoint inhibitors Cytokine inhibitors Tyrosine kinase inhibitors CAR-T cell immunotherapy Corticosteroids (≥20 mg/day for ≥4 weeks) Alkylating agents Anti-proliferative agents Calcineurin inhibitors mTOR inhibitors Janus kinase (JAK) inhibitors | Janus Kinase (JAK) inhibitors |
| Moderate (1–10%) | T-cell-depleting agents Anti-TNF (without steroids) + Anti-rejection (without steroids) | Anti-CD20 **,+ Cytokine inhibitors CAR-T cell immunotherapy Corticosteroids (≥20 mg/day for ≥4 weeks) Calcineurin inhibitors Hematopoietic stem cell transplant + |
| Low (<1%) | Methotrexate Azathioprine | Anti-TNF agents ++ Immune checkpoint inhibitors Tyrosine kinase inhibitors T-cell-depleting agents Alkylating agents |
| Rare | Methotrexate Azathioprine Cytotoxic chemotherapy without steroids | |
| Unknown | mTOR inhibitors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anvari, S.; Tsoi, K. Hepatitis B Virus Reactivation with Immunosuppression: A Hidden Threat? J. Clin. Med. 2024, 13, 393. https://doi.org/10.3390/jcm13020393
Anvari S, Tsoi K. Hepatitis B Virus Reactivation with Immunosuppression: A Hidden Threat? Journal of Clinical Medicine. 2024; 13(2):393. https://doi.org/10.3390/jcm13020393
Chicago/Turabian StyleAnvari, Sama, and Keith Tsoi. 2024. "Hepatitis B Virus Reactivation with Immunosuppression: A Hidden Threat?" Journal of Clinical Medicine 13, no. 2: 393. https://doi.org/10.3390/jcm13020393