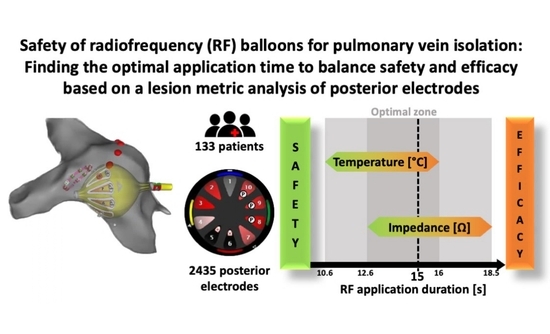
Safety of the Radiofrequency Balloon for Pulmonary Vein Isolation: A Focus on Lesion Metric Analysis of Posterior Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. PVI with the RFB
2.3. RFB Architecture and Lesion Metrics
2.4. Esophageal Temperature Monitoring (ETM)
2.5. Analytics and Statistics
- Baseline impedance (Zbase) and temperature (Tbase),
- Times to reach each impedance drop (ΔZ90%, ΔZ95%, ΔZ100%) and temperature rise (ΔT90%, ΔT95%, ΔT100%) percentiles,
- Absolute impedance at 15 s, (Z(15)90%, Z(15)95%, Z(15)100%) and temperature (T(15)90%, T(15)95%, T(15)100%) values,
- Impedance drop at 15 s, (ΔZ(15)90%, ΔZ(15)95%, ΔZ(15)100%) and temperature rise (ΔT(15)90%, ΔT(15)95%, ΔT(15)100%),
- Impedance drop at 20 s (ΔZ(20)90%, ΔZ(20)95%, ΔZ(20)100%) and temperature rise (ΔT(20)90%, ΔT(20)95%, ΔT(20)100%).
3. Results
3.1. Lesion Metrics
3.2. Lesion Metrics in Posterior Electrodes vs. Esophageal Temperature Monitoring Analysis
4. Discussion
4.1. PVI and Esophageal Injury in Context
4.2. Optimal RF Delivery Time for Best Lesion Metrics
4.3. Optimal Indicators to Spare the Esophagus Temperature rise
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 Year Trends in Atrial Fibrillation Prevalence, Incidence, Risk Factors, and Mortality in the Framingham Heart Study: A Cohort Study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Brugada, J.; Hindricks, G.; Maggioni, A.P.; Tavazzi, L.; Vardas, P.; Laroche, C.; Anselme, F.; Inama, G.; Jais, P.; et al. The Atrial Fibrillation Ablation Pilot Study: An European Survey on Methodology and Results of Catheter Ablation for Atrial Fibrillation Conducted by the European Heart Rhythm Association. Eur. Heart J. 2014, 35, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Tsiachris, D.; Doundoulakis, I.; Antoniou, C.K.; Pagkalidou, E.; Zafeiropoulos, S.; Kordalis, A.; Gatzoulis, K.A.; Chierchia, G.B.; de Asmundis, C.; Tsioufis, K.; et al. Effectiveness and Safety of a Time to Isolation Strategy of Cryoballoon Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2022, 33, 2640–2648. [Google Scholar] [CrossRef]
- Ciconte, G.; De Asmundis, C.; Sieira, J.; Conte, G.; Di Giovanni, G.; Mugnai, G.; Saitoh, Y.; Baltogiannis, G.; Irfan, G.; Coutiño-Moreno, H.E.; et al. Single 3-Minute Freeze for Second-Generation Cryoballoon Ablation: One-Year Follow-up after Pulmonary Vein Isolation. Heart Rhythm. 2015, 12, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.R.J.; Okumura, K.; Scazzuso, F.; Keun On, Y.; Kueffer, F.J.; Braegelmann, K.M.; Kaur Khelae, S.; Al-Kandari, F.; Földesi, C. Safety and Efficacy of Cryoballoon Ablation for the Treatment of Paroxysmal and Persistent AF in a Real-World Global Setting: Results from the Cryo AF Global Registry. J. Arrhythm. 2021, 37, 356–367. [Google Scholar] [CrossRef]
- Almorad, A.; Chierchia, G.B.; Pannone, L.; Osorio, T.G.; Sorgente, A.; Bisignani, A.; Bala, G.; Overeinder, I.; Ströker, E.; Brugada, P.; et al. The Optimized Clinical Workflow for Pulmonary Vein Isolation with the Radiofrequency Balloon. J. Interv. Card. Electrophysiol. 2021, 64, 531–538. [Google Scholar] [CrossRef]
- Monte, A.D.; Almorad, A.; Pannone, L.; Rocca, D.G.D.; Bisignani, A.; Monaco, C.; Mouram, S.; Ramak, R.; Gauthey, A.; Overeinder, I.; et al. Pulmonary Vein Isolation with the Radiofrequency Balloon Catheter: A Single Centre Prospective Study. Europace 2023, 25, 896. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Schilling, R.; Grimaldi, M.; Horton, R.; Natale, A.; Riva, S.; Tondo, C.; Kuck, K.H.; Neuzil, P.; Mcinnis, K.; et al. Pulmonary Vein Isolation With a Novel Multielectrode Radiofrequency Balloon Catheter That Allows Directionally Tailored Energy Delivery: Short-Term Outcomes from a Multicenter First-in-Human Study (RADIANCE). Circ. Arrhythm. Electrophysiol. 2019, 12, e007541. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Honarbakhsh, S.; Di Monaco, A.; Coling, A.E.; Lenka, K.; Pizzamiglio, F.; Hunter, R.J.; Horton, R.; Mansour, M.; Natale, A.; et al. Use of a Multi-Electrode Radiofrequency Balloon Catheter to Achieve Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation: 12-Month Outcomes of the RADIANCE Study. J. Cardiovasc. Electrophysiol. 2020, 31, 1259–1269. [Google Scholar] [CrossRef]
- Schilling, R.; Dhillon, G.S.; Tondo, C.; Riva, S.; Grimaldi, M.; Quadrini, F.; Neuzil, P.; Chierchia, G.B.; De Asmundis, C.; Abdelaal, A.; et al. Safety, Effectiveness, and Quality of Life Following Pulmonary Vein Isolation with a Multi-Electrode Radiofrequency Balloon Catheter in Paroxysmal Atrial Fibrillation: 1-Year Outcomes from SHINE. EP Eur. 2021, 23, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Barkagan, M.; Rottmann, M.; Leshem, E.; Shen, C.; Buxton, A.E.; Anter, E. Effect of Baseline Impedance on Ablation Lesion Dimensions. Circ. Arrhythm. Electrophysiol. 2018, 11, e006690. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, B.; Deneke, T.; Turagam, M.; Dar, T.; Paleti, S.; Parikh, V.; DiBiase, L.; Halfbass, P.; Santangeli, P.; Mahapatra, S.; et al. Temporal Relationships between Esophageal Injury Type and Progression in Patients Undergoing Atrial Fibrillation Catheter Ablation. Heart Rhythm. 2019, 16, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; El Haddad, M.; De Wilde, V.; Phlips, T.; De Pooter, J.; Almorad, A.; Strisciuglio, T.; Vandekerckhove, Y.; Tavernier, R.; Crijns, H.J.H.J.; et al. Endoscopic Evaluation of the Esophagus after Catheter Ablation of Atrial Fibrillation Using Contiguous and Optimized Radiofrequency Applications. Heart Rhythm. 2019, 16, 1013–1020. [Google Scholar] [CrossRef]
- Fürnkranz, A.; Bordignon, S.; Böhmig, M.; Konstantinou, A.; Dugo, D.; Perrotta, L.; Klopffleisch, T.; Nowak, B.; Dignaß, A.U.; Schmidt, B.; et al. Reduced Incidence of Esophageal Lesions by Luminal Esophageal Temperature-Guided Second-Generation Cryoballoon Ablation. Heart Rhythm. 2015, 12, 268–274. [Google Scholar] [CrossRef]
- Kanthasamy, V.; Breitenstein, A.; Schilling, R.; Hofer, D.; Tiongco, B.; Ang, R.; Hunter, R.; Earley, M.; Ahsan, S.; Mangiafico, V.; et al. Catheter Ablation of Atrial Fibrillation with a Multi-Electrode Radiofrequency Balloon; First and Early Two Centre Experience in Europe. J. Cardiovasc. Electrophysiol. 2023, 34, 1350–1359. [Google Scholar] [CrossRef]
- Bordignon, S.; My, I.; Tohoku, S.; Rillig, A.; Schaack, D.; Chen, S.; Reißmann, B.; Urbanek, L.; Hirokami, J.; Efe, T.; et al. Efficacy and Safety in Patients Treated with a Novel Radiofrequency Balloon: A Two Centres Experience from the AURORA Collaboration. Europace 2023, 25, euad106. [Google Scholar] [CrossRef]
- Santoro, A.; Baiocchi, C.; Lumia, G.; Zacà, V.; Romano, A.; Spera, L.; Stricagnoli, M.; Falciani, F.; Valente, S.; Gaspardone, A.; et al. Detection of Oesophageal Course during Left Atrial Catheter Ablation. Indian Pacing Electrophysiol. J. 2020, 20, 221–226. [Google Scholar] [CrossRef]
- Tilz, R.R.; Schmidt, V.; Pürerfellner, H.; Maury, P.; Chun, K.J.; Martinek, M.; Sohns, C.; Schmidt, B.; Mandel, F.; Gandjbakhch, E.; et al. A Worldwide Survey on Incidence, Management and Prognosis of Oesophageal Fistula Formation Following Atrial Fibrillation Catheter Ablation: The POTTER-AF Study. Eur. Heart J. 2023, 44, 2458–2469. [Google Scholar] [CrossRef]
- Suenari, K.; Nakano, Y.; Hirai, Y.; Ogi, H.; Oda, N.; Makita, Y.; Ueda, S.; Kajihara, K.; Tokuyama, T.; Motoda, C.; et al. Left Atrial Thickness under the Catheter Ablation Lines in Patients with Paroxysmal Atrial Fibrillation: Insights from 64-Slice Multidetector Computed Tomography. Heart Vessel. 2013, 28, 360–368. [Google Scholar] [CrossRef]
- Sánchez-Quintana, D.; Cabrera, J.A.; Climent, V.; Farré, J.; De Mendonça, M.C.; Ho, S.Y. Anatomic Relations between the Esophagus and Left Atrium and Relevance for Ablation of Atrial Fibrillation. Circulation 2005, 112, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Chinitz, J.; Michaud, G.; Stephenson, K. Impedance-Guided Radiofrequency Ablation: Using Impedance to Improve Ablation Outcomes. J. Innov. Card Rhythm. Manag. 2017, 8, 2868. [Google Scholar] [CrossRef] [PubMed]
- Avitall, B.; Mughal, K.; Hare, J.; Helms, R.; Krum, D. The Effects of Electrode-Tissue Contact on Radiofrequency Lesion Generation. PACE—Pacing Clin. Electrophysiol. 1997, 20, 2899–2910. [Google Scholar] [CrossRef] [PubMed]





| Metrics | Baseline | 90% | 95% | Plateau | |
|---|---|---|---|---|---|
| Impedance | Z (Ω) | 102.4 (95.8–111.1) | 86.6 (81.3.–92) | 85.6 (80.4–91.1) | 84.6 (79.3–90.2) |
| ΔZ (Ω) | NA | 16.6 (11–22.7) | 17.5 (11.6–24) | 18.4 (12.2–25.2) | |
| Time (s) | NA | 10.2 (7.9–11.9) | 11.8 (9.1–13.8) | 13.9 (10.6–16) | |
| Electrodes’ distribution * | NA | 99.6% | 95.8% | 75.6% | |
| Temperature | T (°C) | 27.1 (25.8–28.9) | 34.2 (31.8–37.3) | 36.1 (33.5–39.3) | 38 (35.3–41.4) |
| ΔT (°C) | NA | 7.3 (3.7–10.8) | 9.1 (5.3–12.8) | 11.1 (7.1–14.9) | |
| Time (s) | NA | 7.2 (3.9–9.6) | 10.1 (6.4–13.1) | 16.4 (12.6–18.4) | |
| Electrodes’ distribution ** | NA | 97.2 % | 92.8% | 47% |
| Baseline | 90% | 95% | Plateau | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | ETR − | ETR + | p Value | ETR − | ETR + | p Value | ETR − | ETR + | p Value | ETR − | ETR + | p Value | |
| Impedance | Z (Ω) | 101.8 (95.5–110.5) | 105.1 (98.3–115.5) | 0.018 | 86.6 (81.4–92.2) | 86.6 (79.6–90.6) | 0.071 | 85.6 (80.5–91.4) | 83.5 (78.4–89.3) | 0.031 | 84.8 (79.4–90.6) | 82.2 (77.1–87.5) | 0.012 |
| ΔZ (20) (Ω) | NA | NA | NA | 16 (10.1–22.1) | 21.4 (15–28.5) | 0.0001 | 16.9 (10.7–23.4) | 22.6 (15.8–30) | 0.0001 | 17.8 (11.2–24.6) | 23.8 (16.7–31.7) | 0.0001 | |
| Time (s) | NA | NA | NA | 10 (7.5–11.8) | 10.4 (8.8–12) | 0.048 | 11.5 (8.7–13.7) | 12.5 (10.6–13.7) | 0.009 | 13.6 (10–15,9) | 14.7 (12.3–16.4) | 0.005 | |
| Temperature | T (°C) | 27.3 (26–29) | 26.1 (25.1–28.1) | 0.0001 | 34.2 (31.8–37.2) | 26.1 (25.1–28.1) | 0.001 | 36.1 (33.5–39.2) | 37 (34.5–40.8) | 0.002 | 38 (35.3–41.3) | 39 (36.3–42.9) | 0.002 |
| ΔT (20) (°C) | NA | NA | NA | 7.1 (3.5–10.7) | 9.1 (6.4–12.5) | 0.0001 | 8.9 (5.1–12.7) | 11.3 (8.1–14.7) | 0.002 | 10.8 (6.9–14.8) | 13.1 (9.9–16.9) | 0.0001 | |
| Time (s) | NA | NA | NA | 7.1 (3.6–9.5) | 8.6 (5.9–10.5) | 0.0007 | 10 (6–13) | 11.3 (8.7–14.2) | 0.004 | 16.3 (12.3–18.4) | 13.6 (10–15.9) | 0.47 | |
| Baseline | 90% | 95% | Plateau | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | ETR − | ETR + | p Value | ETR − | ETR + | p Value | ETR − | ETR + | p Value | ETR − | ETR + | p Value | |
| Impedance | Z (15) (Ω) | 86.6 (81.4–92.2) | 85 (79.6–90.6) | 0.02 | 85.7 (81.4–92.2) | 83 (78.4–88.2) | 0.01 | 85.2 (79.9–91.1) | 81.5 (77.3–86.0) | 0.001 | 86.6 (81.4–92.2) | 85 (79.6–90.6) | 0.02 |
| ΔZ (15) (Ω) | 16 (10.1–22.1) | 21.4 (15–28.5) | 0.0001 | 16.5 (10.5–22.5) | 22.2 (15.7–28.4) | 0.0001 | 15.4 (9.7–21.5) | 22.3 (16.1–27.6) | 0.0001 | 16 (10.1–22.1) | 21.4 (15–28.5) | 0.0001 | |
| Electrodes distribution * | 99.5% | 100% | 92.3% | 95.8% | 76.4% | 71.8% | 99.5% | 100% | |||||
| Temperature | T (15) (°C) | 34.2 (31.7–31.7) | 35.1 (32.7–32.7) | 0.034 | 35.7 (33.4–33.4) | 36.8 (34.3–34.3) | 0.034 | 35.9 (34.5–34.5) | 37.4 (35.7–35.7) | 0.034 | 34.2 (31.7–31.7) | 35.1 (32.7–32.7) | 0.034 |
| ΔT at 15 s | 7 (3.5–10.6) | 9.1 (6.4–12.5) | 0.001 | 8.5 (4.9–11.9) | 10.9 (7.3–14.2) | 0.001 | 7.6 (5–11.3) | 11.2 (7–14.5) | 0.003 | 7 (3.5–10.6) | 9.1 (6.4–12.5) | 0.001 | |
| Electrodes distribution * | 97.3% | 100% | 92.6% | 93.6% | 47.4% | 46.1% | 97.3% | 100% | |||||
| At 15 s | At 20 s | p Value | |
|---|---|---|---|
| ΔT100% (°C) | 11.2 (7–14.5) | 13.1 (9.9–16.8) | 0.11 |
| ΔΩ100% (Ω) | 22.3 (16.1–27.6) | 23.8 (16.7–31.7) | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almorad, A.; Del Monte, A.; Teumer, Y.; El Haddad, M.; Pannone, L.; Della Rocca, D.G.; Audiat, C.; Cespón-Fernández, M.; Mouram, S.; Ramak, R.; et al. Safety of the Radiofrequency Balloon for Pulmonary Vein Isolation: A Focus on Lesion Metric Analysis of Posterior Electrodes. J. Clin. Med. 2023, 12, 6256. https://doi.org/10.3390/jcm12196256
Almorad A, Del Monte A, Teumer Y, El Haddad M, Pannone L, Della Rocca DG, Audiat C, Cespón-Fernández M, Mouram S, Ramak R, et al. Safety of the Radiofrequency Balloon for Pulmonary Vein Isolation: A Focus on Lesion Metric Analysis of Posterior Electrodes. Journal of Clinical Medicine. 2023; 12(19):6256. https://doi.org/10.3390/jcm12196256
Chicago/Turabian StyleAlmorad, Alexandre, Alvise Del Monte, Yannick Teumer, Milad El Haddad, Luigi Pannone, Domenico Giovanni Della Rocca, Charles Audiat, María Cespón-Fernández, Sahar Mouram, Robbert Ramak, and et al. 2023. "Safety of the Radiofrequency Balloon for Pulmonary Vein Isolation: A Focus on Lesion Metric Analysis of Posterior Electrodes" Journal of Clinical Medicine 12, no. 19: 6256. https://doi.org/10.3390/jcm12196256