Microneedle Arrays Combined with Nanomedicine Approaches for Transdermal Delivery of Therapeutics
Abstract
:1. Introduction
2. MNs: An Advancement in Hypodermic Needles
- (A)
- Poke-and-patch approach to apply tens to tens of thousands of MNs as a pore-forming pretreatment. Afterward, a conventional drug formulation is applied on the skin surface.
- (B)
- Coat-and-poke approach, which consists of a water-soluble drug coating on solid MNs. Drug coating is dissolved and simply deposited within the skin during its administration.
- (C)
- Poke-and-release approach in which water-insoluble MNs are injected into the skin. The encapsulated therapeutic agent is slowly released, while the patch remains on the skin after its application.
- (D)
- Poke-and-flow approach, which is characterized by a hole in the structure (center or side) of each microneedle to enable the drug flow across the skin.
- (E)
- Poke-and-dissolve approach, which uses biodegradable or water-soluble drug-encapsulated MNs. The MNs dissolve and release their loaded therapeutic agents into the skin. Table 1 summarizes different delivery strategies, advantages, disadvantages, and applications of MNs.
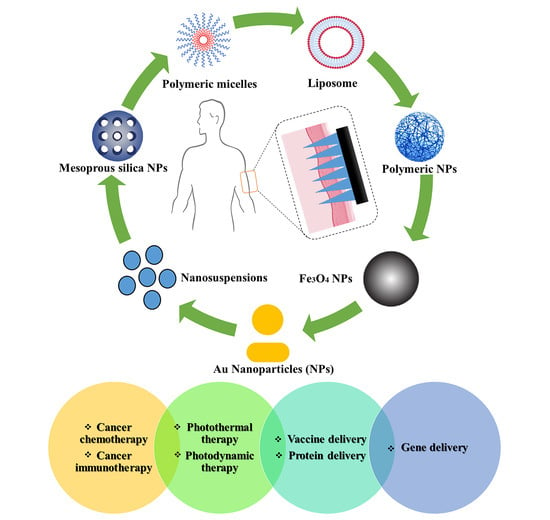
3. Potential Applications of MN Combination with NPs
3.1. MN-Assisted NP Delivery in Cancer Chemotherapy
3.2. MN-Assisted NP Delivery in Cancer Immunotherapy
3.3. MN-Assisted NP Delivery in Photothermal Therapy
3.4. MN-Assisted NP Delivery in Photodynamic Therapy
3.5. MN-Assisted NP Delivery in Delivery of Therapeutic Proteins
3.6. MN-Assisted NP Delivery in Vaccine Delivery
3.7. MN-Assisted NP Delivery for Gene Therapy
4. Advances in Clinical Trials and Commercialization of MNs
5. Current Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Boukherroub, R. Heat: A Highly Efficient Skin Enhancer for Transdermal Drug Delivery. Front. Bioeng. Biotechnol. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261. [Google Scholar] [CrossRef] [PubMed]
- Moradi, L.; Javanmardi, S.; Abolmaali, S.; Mohammadi Samani, S. Passive Enhancement of Transdermal Drug Delivery: Lipid-Based Colloidal Carriers as an Emerging Pharmaceutical Technology Platform. TiPS 2019, 5, 25–40. [Google Scholar]
- Yang, M.-Y.; Zhao, R.-R.; Fang, Y.-F.; Jiang, J.-L.; Yuan, X.-T.; Shao, J.-W. Carrier-free nanodrug: A novel strategy of cancer diagnosis and synergistic therapy. Int. J. Pharm. 2019, 570, 118663. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, F.; Chen, D.; Wang, Y.; Tan, Y.; Ban, J. Transdermal permeability of triamcinolone acetonide lipid nanoparticles. Int. J. Nanomed. 2019, 14, 2485–2495. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, H.; Aqil, M.; Imam, S.S.; Sultana, Y.; Ali, A. Formulation of amlodipine nano lipid carrier: Formulation design, physicochemical and transdermal absorption investigation. J. Drug Deliv. Sci. Technol. 2019, 49, 209–218. [Google Scholar] [CrossRef]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef]
- Abolmaali, S.; Tamaddon, A.; Salmanpour, M.; Mohammadi, S.; Dinarvand, R. Block ionomer micellar nanoparticles from double hydrophilic copolymers, classifications and promises for delivery of cancer chemotherapeutics. Eur. J. Pharm. Sci. 2017, 104, 393–405. [Google Scholar] [CrossRef]
- Abolmaali, S.S.; Tamaddon, A.M.; Dinarvand, R. Nano-hydrogels of methoxy polyethylene glycol-grafted branched polyethyleneimine via biodegradable cross-linking of Zn2+-ionomer micelle template. J. Nanopart. Res. 2013, 15, 2134. [Google Scholar] [CrossRef]
- Labouta, H.I.; Schneider, M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomed. Nanotechnol. 2013, 9, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.; Alimardani, V.; Roudbali, P.L.; Ghasemi, Y.; Kaviani, E. Gold nanoparticles application in liver cancer. Photodiagn. Photodyn. Ther. 2019, 25, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Dhal, S.; Pal, K.; Giri, S. Transdermal Delivery of Gold Nanoparticles by a Soybean Oil-Based Oleogel under Iontophoresis. ACS Appl. Bio Mater. 2020, 3, 7029–7039. [Google Scholar] [CrossRef]
- Larese Filon, F.; Crosera, M.; Adami, G.; Bovenzi, M.; Rossi, F.; Maina, G. Human skin penetration of gold nanoparticles through intact and damaged skin. Nanotoxicology 2011, 5, 493–501. [Google Scholar] [CrossRef]
- Rangsimawong, W.; Opanasopit, P.; Rojanarata, T.; Panomsuk, S.; Ngawhirunpat, T. Influence of sonophoresis on transdermal drug delivery of hydrophilic compound-loaded lipid nanocarriers. Pharm. Dev. Technol. 2017, 22, 597–605. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Liu, C.-G. Polymeric nanoparticles based on carboxymethyl chitosan in combination with painless microneedle therapy systems for enhancing transdermal insulin delivery. RSC Adv. 2020, 10, 24319–24329. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the clinic. J. Control. Release 2017, 260, 164–182. [Google Scholar] [CrossRef]
- Alimardani, V.; Abolmaali, S.S.; Tamaddon, A.M.; Ashfaq, M. Recent advances on microneedle arrays-mediated technology in cancer diagnosis and therapy. Drug Deliv. Transl. Res. 2020, 1–29. [Google Scholar] [CrossRef]
- Dsouza, L.; Ghate, V.M.; Lewis, S.A. Derma rollers in therapy: The transition from cosmetics to transdermal drug delivery. Biomed. Microdevices 2020, 22, 1–11. [Google Scholar] [CrossRef]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Castañeda, P.; Escobar-Chavez, J.J.; Rodríguez-Cruz, I.M.; Melgoza, L.M.; Martinez-Hernandez, J. Microneedles as Enhancer of Drug Absorption Through the Skin and Applications in Medicine and Cosmetology. J. Pharm. Pharm. Sci. 2018, 21, 73–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larraneta, E.; Lutton, R.E.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Prausnitz, M.R. Engineering microneedle patches for vaccination and drug delivery to skin. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharm. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Lim, D.-J.; Vines, J.B.; Park, H.; Lee, S.-H. Microneedles: A versatile strategy for transdermal delivery of biological molecules. Int. J. Biol. Macromol. 2018, 110, 30–38. [Google Scholar] [CrossRef]
- van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans) dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef]
- Jung, J.H.; Chiang, B.; Grossniklaus, H.E.; Prausnitz, M.R. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J. Control. Release 2018, 277, 14–22. [Google Scholar] [CrossRef]
- DeMuth, P.C.; Moon, J.J.; Suh, H.; Hammond, P.T.; Irvine, D.J. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano 2012, 6, 8041–8051. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, P.C.; Su, X.; Samuel, R.E.; Hammond, P.T.; Irvine, D.J. Nano-layered microneedles for transcutaneous delivery of polymer nanoparticles and plasmid DNA. Adv. Mater. 2010, 22, 4851–4856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, P.; van der Maaden, K.; Groeneveld, V.; Ruigrok, M.; Romeijn, S.; Uleman, S.; Oomens, C.; Kersten, G.; Jiskoot, W.; Bouwstra, J. Diphtheria toxoid and N-trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J. Control. Release 2017, 262, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ingrole, R.S.; Gill, H.S. Microneedle coating methods: A review with a perspective. J. Pharmacol. Exp. Ther. 2019, 370, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Gadeela, P.R.; Thathireddy, P.; Venuganti, V.V.K. Microneedle-based drug delivery: Materials of construction. J. Chem. Sci. 2019, 131, 90. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Polymer Microneedles for Controlled-Release Drug Delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhu, D.D.; Liu, X.B.; Chen, B.Z.; Guo, X.D. Microneedles with controlled bubble sizes and drug distributions for efficient transdermal drug delivery. Sci. Rep. 2016, 6, 38755. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.Y.; Prausnitz, M.R. Separable arrowhead microneedles. J. Control. Release 2011, 149, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Xue, P.; Zhang, X.; Chuah, Y.J.; Wu, Y.; Kang, Y. Flexible PEGDA-based microneedle patches with detachable PVP–CD arrowheads for transdermal drug delivery. RSC Adv. 2015, 5, 75204–75209. [Google Scholar] [CrossRef]
- Zhu, D.D.; Wang, Q.L.; Liu, X.B.; Guo, X.D. Rapidly separating microneedles for transdermal drug delivery. Acta Biomater. 2016, 41, 312–319. [Google Scholar] [CrossRef]
- Juster, H.; van der Aar, B.; de Brouwer, H. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym. Eng. Sci. 2019, 59, 877–890. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Carrier, A.; Chen, Y.; Lin, S.; Wang, J.; Cui, S.; Zhang, X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release 2019, 315, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-J.; Lin, Y.-J.; Hu, Y.-C.; Chiang, W.-L.; Chen, K.-J.; Yang, W.-C.; Liu, H.-L.; Fu, C.-C.; Sung, H.-W. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials 2012, 33, 5156–5165. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, J.; Hu, Q.; Hochu, G.M.; Xin, H.; Wang, C.; Gu, Z. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano 2016, 10, 8956–8963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Lutton, R.E.M.; Brady, A.J.; Vicente-Pérez, E.M.; Woolfson, A.D.; Thakur, R.R.S.; Donnelly, R.F. Microwave-Assisted Preparation of Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery Applications. Macromol. Mater. Eng. 2015, 300, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.G.; Larrañeta, E.; Donnelly, R.F.; McGoldrick, N.; Migalska, K.; McCrudden, M.T.; Irwin, N.J.; Donnelly, L.; McCoy, C.P. Hydrogel-forming microneedle arrays made from light-responsive materials for on-demand transdermal drug delivery. Mol. Pharm. 2016, 13, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Wu, Z.; Chen, L.; Wu, F.; Wei, L.; Yuan, W. Hydrogel microneedle arrays for transdermal drug delivery. Nanomicro Lett. 2014, 6, 191–199. [Google Scholar] [CrossRef]
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater. Sci. Eng. C 2019, 103, 109717. [Google Scholar] [CrossRef]
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.A.; McCrudden, M.T.C.; Kearney, M.-C.; McAlister, E.; McCarthy, H.O.; Donnelly, R.F. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151. [Google Scholar] [CrossRef]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019, 11, 5781–5789. [Google Scholar] [CrossRef]
- Demir, Y.K.; Metin, A.Ü.; Şatıroğlu, B.; Solmaz, M.E.; Kayser, V.; Mäder, K. Poly (methyl vinyl ether-co-maleic acid)-Pectin based hydrogel-forming systems: Gel, film, and microneedles. Eur. J. Pharm. Biopharm. 2017, 117, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, A.; Banga, A.K. Novel in situ forming hydrogel microneedles for transdermal drug delivery. Drug Deliv. Transl. Res. 2017, 7, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Singh, T.R.R.; Woolfson, A.D.; Donnelly, R.F. Electrically enhanced solute permeation across poly (ethylene glycol)–crosslinked poly (methyl vinyl ether-co-maleic acid) hydrogels: Effect of hydrogel crosslink density and ionic conductivity. Int. J. Pharm. 2011, 406, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Zhang, J.N.; Chen, B.Z.; Wang, Q.L.; Guo, X.D. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017, 7, 15408–15415. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Roh, M.R.; PARK, G.h.; Kim, Y.J.; Jeon, I.K.; Chang, S.E. Fractionated microneedle radiofrequency for the treatment of periorbital wrinkles. J. Dermatol. 2013, 40, 172–176. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.M.; Platteel, A.; Kuijt, N.; van Kooten, P.J.; Vos, P.J.; Sijts, A.J.; Van Der Maaden, K. Nanoporous microneedle arrays effectively induce antibody responses against diphtheria and tetanus toxoid. Front. Immunol. 2017, 8, 1789. [Google Scholar] [CrossRef] [Green Version]
- Abiandu, I.; Ita, K. Transdermal delivery of potassium chloride with solid microneedles. J. Drug Deliv. Sci. Technol. 2019, 53, 101216. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive Microneedle-based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Senel, M.; Dervisevic, M.; Voelcker, N.H. Gold microneedles fabricated by casting of gold ink used for urea sensing. Mater. Lett. 2019, 243, 50–53. [Google Scholar] [CrossRef]
- Meyer, B.K.; Kendall, M.A.; Williams, D.M.; Bett, A.J.; Dubey, S.; Gentzel, R.C.; Casimiro, D.; Forster, A.; Corbett, H.; Crichton, M. Immune Response and Reactogenicity of an Unadjuvanted Intradermally Delivered Human Papillomavirus Vaccine Using a First Generation Nanopatch™ in Rhesus Macaques: An Exploratory, Pre-Clinical Feasibility Assessment. Vaccine: X 2019, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Scoutaris, N.; Lamprou, D.; Mallinson, D.; Douroumis, D. Inkjet printing of insulin microneedles for transdermal delivery. Drug Deliv. Transl. Res. 2015, 5, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Maaden, K.; Yu, H.; Sliedregt, K.; Zwier, R.; Leboux, R.; Oguri, M.; Kros, A.; Jiskoot, W.; Bouwstra, J.A. Nanolayered chemical modification of silicon surfaces with ionizable surface groups for pH-triggered protein adsorption and release: Application to microneedles. J. Mater. Chem. B 2013, 1, 4466–4477. [Google Scholar] [CrossRef] [PubMed]
- Cormier, M.; Johnson, B.; Ameri, M.; Nyam, K.; Libiran, L.; Zhang, D.D.; Daddona, P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 2004, 97, 503–511. [Google Scholar] [CrossRef]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.-F. Parathyroid Hormone (1-34)-Coated Microneedle Patch System: Clinical Pharmacokinetics and Pharmacodynamics for Treatment of Osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef]
- Al Sulaiman, D.; Chang, J.Y.H.; Bennett, N.R.; Topouzi, H.; Higgins, C.A.; Irvine, D.J.; Ladame, S. Hydrogel-Coated Microneedle Arrays for Minimally Invasive Sampling and Sensing of Specific Circulating Nucleic Acids from Skin Interstitial Fluid. ACS Nano 2019, 13, 9620–9628. [Google Scholar] [CrossRef]
- van der Maaden, K.; Heuts, J.; Camps, M.; Pontier, M.; van Scheltinga, A.T.; Jiskoot, W.; Ossendorp, F.; Bouwstra, J. Hollow microneedle-mediated micro-injections of a liposomal HPV E743–63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control. Release 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Zu, Q.; Yu, Y.; Bi, X.; Zhang, R.; Di, L. Microneedle-Assisted Percutaneous Delivery of a Tetramethylpyrazine-Loaded Microemulsion. Molecules 2017, 22, 2022. [Google Scholar] [CrossRef] [Green Version]
- Iliescu, F.S.; Teo, J.C.M.; Vrtacnik, D.; Taylor, H.; Iliescu, C. Cell therapy using an array of ultrathin hollow microneedles. Microsyst.Technol. 2018, 24, 2905–2912. [Google Scholar] [CrossRef]
- Golombek, S.; Pilz, M.; Steinle, H.; Kochba, E.; Levin, Y.; Lunter, D.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Intradermal Delivery of Synthetic mRNA Using Hollow Microneedles for Efficient and Rapid Production of Exogenous Proteins in Skin. Mol. Ther. Nucleic Acids. 2018, 11, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Dul, M.; Stefanidou, M.; Porta, P.; Serve, J.; O’Mahony, C.; Malissen, B.; Henri, S.; Levin, Y.; Kochba, E.; Wong, F.S.; et al. Hydrodynamic gene delivery in human skin using a hollow microneedle device. J. Control. Release 2017, 265, 120–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Tay, F.; Guo, D.; Xu, L.; Yap, K. A microfabricated electrode with hollow microneedles for ECG measurement. Sens. Actuators A 2009, 151, 17–22. [Google Scholar] [CrossRef]
- Daugimont, L.; Baron, N.; Vandermeulen, G.; Pavselj, N.; Miklavcic, D.; Jullien, M.-C.; Cabodevila, G.; Mir, L.M.; Préat, V. Hollow microneedle arrays for intradermal drug delivery and DNA electroporation. J. Membr. Biol. 2010, 236, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, D.; Logan, K.A.; Sheng, Y.; Gao, J.; Farrell, S.; Dixon, D.; Callan, B.; McHale, A.P.; Callan, J.F. Rapid paper based colorimetric detection of glucose using a hollow microneedle device. Int. J. Pharm. 2018, 547, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lai, K.-Y.; Chiu, Y.-H.; Wu, Y.-W.; Shiau, A.-L.; Chen, M.-C. Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination. Acta Biomater. 2019, 97, 230–238. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Kim, N.W.; Thambi, T.; Giang Phan, V.H.; Lee, M.S.; Yin, Y.; Jeong, J.H.; Lee, D.S. Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J. Control. Release 2018, 269, 225–234. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Li, Z.; Xu, N.; Liu, P.; Du, H.; Zhang, Y.; Huang, Y.; Zhu, J.; Ren, G. Au nanocage-strengthened dissolving microneedles for chemo-photothermal combined therapy of superficial skin tumors. ACS Appl. Mater. Interfaces 2018, 10, 9247–9256. [Google Scholar] [CrossRef]
- Dillon, C.; Hughes, H.; O’Reilly, N.J.; Allender, C.J.; Barrow, D.A.; McLoughlin, P. Dissolving microneedle based transdermal delivery of therapeutic peptide analogues. Int. J. Pharm. 2019, 565, 9–19. [Google Scholar] [CrossRef]
- Lin, S.; Quan, G.; Hou, A.; Yang, P.; Peng, T.; Gu, Y.; Qin, W.; Liu, R.; Ma, X.; Pan, X.; et al. Strategy for hypertrophic scar therapy: Improved delivery of triamcinolone acetonide using mechanically robust tip-concentrated dissolving microneedle array. J. Control. Release 2019, 306, 69–82. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef]
- Huh, I.; Kim, S.; Yang, H.; Jang, M.; Kang, G.; Jung, H. Effects of two droplet-based dissolving microneedle manufacturing methods on the activity of encapsulated epidermal growth factor and ascorbic acid. Eur. J. Pharm. Sci. 2018, 114, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, S.; Jang, M.; Kim, H.; Lee, S.; Kim, Y.; Eom, Y.A.; Kang, G.; Chiang, L.; Baek, J.H.; et al. Two-phase delivery using a horse oil and adenosine-loaded dissolving microneedle patch for skin barrier restoration, moisturization, and wrinkle improvement. J. Cosmet. Dermatol. 2019, 18, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Ramöller, I.K.; Tekko, I.A.; McCarthy, H.O.; Donnelly, R.F. Rapidly dissolving bilayer microneedle arrays–A minimally invasive transdermal drug delivery system for vitamin B12. Int. J. Pharm. 2019, 566, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, I.M.N.; Tekko, I.A.; Matchett, K.B.; Arnaut, L.G.; Silva, C.S.; McCarthy, H.O.; Donnelly, R.F. Intradermal Delivery of a Near-Infrared Photosensitizer Using Dissolving Microneedle Arrays. J. Pharm. Sci. 2018, 107, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, F.; Su, C.; Yu, B.; Liu, D.; Chen, H.-J.; Lin, D.-a.; Yang, C.; Zhou, L.; Wu, Q.; et al. Biodegradable Therapeutic Microneedle Patch for Rapidly Antihypertensive Treatment. ACS Appl. Mater. Interfaces 2019, 11, 30575–30584. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31. [Google Scholar] [CrossRef]
- Courtenay, A.J.; Rodgers, A.M.; McCrudden, M.T.C.; McCarthy, H.O.; Donnelly, R.F. Novel Hydrogel-Forming Microneedle Array for Intradermal Vaccination in Mice Using Ovalbumin as a Model Protein Antigen. Mol. Pharm. 2019, 16, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Eltayib, E.; Brady, A.J.; Caffarel-Salvador, E.; Gonzalez-Vazquez, P.; Zaid Alkilani, A.; McCarthy, H.O.; McElnay, J.C.; Donnelly, R.F. Hydrogel-forming microneedle arrays: Potential for use in minimally-invasive lithium monitoring. Eur. J. Pharm. Biopharm. 2016, 102, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Caffarel-Salvador, E.; Brady, A.J.; Eltayib, E.; Meng, T.; Alonso-Vicente, A.; Gonzalez-Vazquez, P.; Torrisi, B.M.; Vicente-Perez, E.M.; Mooney, K.; Jones, D.S.; et al. Hydrogel-Forming Microneedle Arrays Allow Detection of Drugs and Glucose In Vivo: Potential for Use in Diagnosis and Therapeutic Drug Monitoring. PLoS ONE 2016, 10, e0145644. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.; Larrañeta, E.; McCrudden, M.T.; McCrudden, C.M.; Brady, A.J.; Fallows, S.J.; McCarthy, H.O.; Kissenpfennig, A.; Donnelly, R.F. In vivo studies investigating biodistribution of nanoparticle-encapsulated rhodamine B delivered via dissolving microneedles. J. Control. Release 2017, 265, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Abedi, M.; Abolmaali, S.S.; Abedanzadeh, M.; Borandeh, S.; Samani, S.M.; Tamaddon, A.M. Citric acid functionalized silane coupling versus post-grafting strategy for dual pH and saline responsive delivery of cisplatin by Fe3O4/carboxyl functionalized mesoporous SiO2 hybrid nanoparticles: A-synthesis, physicochemical and biological characterization. Mater. Sci. Eng. C 2019, 104, 109922. [Google Scholar]
- Abedi, M.; Abolmaali, S.S.; Abedanzadeh, M.; Farjadian, F.; Samani, S.M.; Tamaddon, A.M. Core–Shell Imidazoline–Functionalized Mesoporous Silica Superparamagnetic Hybrid Nanoparticles as a Potential Theranostic Agent for Controlled Delivery of Platinum (II) Compound. Int. J. Nanomed. 2020, 15, 2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navya, P.; Daima, H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016, 3, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Patravale, V.; Dandekar, P.; Jain, R. Nanoparticulate Drug Delivery: Perspectives on the Transition from Laboratory to Market; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Xie, S.; Li, Z.; Yu, Z. Microneedles for transdermal delivery of insulin. J. Drug Deliv. Sci. Technol. 2015, 28, 11–17. [Google Scholar] [CrossRef]
- Chu, L.-Y. Controlled release systems for insulin delivery. Expert Opin. Ther. Pat. 2005, 15, 1147–1155. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Fallows, S.J.; Birkhäuer, L.L.; McCrudden, M.T.C.; Woolfson, A.D.; Donnelly, R.F. A facile system to evaluate in vitro drug release from dissolving microneedle arrays. Int. J. Pharm. 2016, 497, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, E.; Borg, T.; Abdelghani, G.; Saleh, N.M. Transdermal microneedle-mediated delivery of polymeric lamivudine-loaded nanoparticles. J. Pharm. Tech. Drug Res. 2016, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-G.; Gater, D.L.; Kim, Y.-C. Development of transdermal vitamin D3 (VD3) delivery system using combinations of PLGA nanoparticles and microneedles. Drug Deliv. Transl. Res. 2018, 8, 281–290. [Google Scholar] [CrossRef]
- Cui, Y.; Mo, Y.; Zhang, Q.; Tian, W.; Xue, Y.; Bai, J.; Du, S. Microneedle-Assisted Percutaneous Delivery of Paeoniflorin-Loaded Ethosomes. Molecules 2018, 23, 3371. [Google Scholar] [CrossRef] [Green Version]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelchen, M.N.; Brogden, N.K. In Vitro Skin Retention and Drug Permeation through Intact and Microneedle Pretreated Skin after Application of Propranolol Loaded Microemulsions. Pharm. Res. 2018, 35, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Yu, J.; Yu, S.; Wang, J.; Qiang, L.; Gu, Z. Locally Induced Adipose Tissue Browning by Microneedle Patch for Obesity Treatment. ACS Nano 2017, 11, 9223–9230. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, Y.; Li, Z.; Zhao, J.; Feng, N. Microneedle-mediated transdermal delivery of nanostructured lipid carriers for alkaloids from Aconitum sinomontanum. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1541–1551. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.-C. Topical delivery of 5-fluorouracil-loaded carboxymethyl chitosan nanoparticles using microneedles for keloid treatment. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Justin, R.; Chen, B. Multifunctional chitosan–magnetic graphene quantum dot nanocomposites for the release of therapeutics from detachable and non-detachable biodegradable microneedle arrays. Interface Focus 2018, 8, 20170055. [Google Scholar] [CrossRef] [Green Version]
- Vora, L.K.; Donnelly, R.F.; Larrañeta, E.; González-Vázquez, P.; Thakur, R.R.S.; Vavia, P.R. Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: Proof of concept. J. Control. Release 2017, 265, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Cao, Q.; Zhang, Y.; Yu, W.; Zhu, J.; Liu, D.; Jiang, G. Microneedles Integrated with ZnO Quantum-Dot-Capped Mesoporous Bioactive Glasses for Glucose-Mediated Insulin Delivery. ACS Biomater. Sci. Eng. 2018, 4, 2473–2483. [Google Scholar] [CrossRef]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. 2020, 2, 119220. [Google Scholar] [CrossRef]
- Permana, A.D.; McCrudden, M.T.; Donnelly, R.F. Enhanced intradermal delivery of nanosuspensions of antifilariasis drugs using dissolving microneedles: A proof of concept study. Pharmaceutics 2019, 11, 346. [Google Scholar] [CrossRef] [Green Version]
- Mir, M.; Permana, A.D.; Ahmed, N.; Khan, G.M.; ur Rehman, A.; Donnelly, R.F. Enhancement in site-specific delivery of carvacrol for potential treatment of infected wounds using infection responsive nanoparticles loaded into dissolving microneedles: A proof of concept study. Eur. J. Pharm. Biopharm. 2020, 147, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Choi, J.-T.; Kim, C.B.; Shin, Y.-R.; Park, P.-G.; Kim, H.; Lee, J.M.; Park, J.-H. Microneedle Array Patch (MAP) Consisting of Crosslinked Hyaluronic Acid Nanoparticles for Processability and Sustained Release. Pharm. Res. 2020, 37, 50. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.; Tekko, I.A.; Vora, L.; Larrañeta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-based dissolving microneedle arrays for intradermal delivery of curcumin. Pharmaceutics 2019, 11, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Chen, Y.; He, X.; Yang, F.; Han, R.; Yang, C.; Li, W.; Qian, Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lin, H.; Wang, Z.; Yang, X.; Zhang, M.; Liu, X.; Wang, B.; Wu, Z.; Chen, D. Preparation and characterization of dissolving hyaluronic acid composite microneedles loaded micelles for delivery of curcumin. Drug Deliv. Transl. Res. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Tekko, I.A.; McCrudden, M.T.; Anjani, Q.K.; Ramadon, D.; McCarthy, H.O.; Donnelly, R.F. Solid lipid nanoparticle-based dissolving microneedles: A promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis. J. Control. Release 2019, 316, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, N.; Wang, J.; Tung, M.; Conway, C.; Chung, E. Transdermal Delivery of Kidney Targeting Nanoparticles Using Dissolvable Microneedles. Cell. Mol. Bioeng. 2020, 13, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, B.; Park, J.-H. Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin. Biomaterials 2012, 33, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Girma, W.M.; Tzing, S.-H.; Tseng, P.-J.; Huang, C.-C.; Ling, Y.-C.; Chang, J.-Y. Synthesis of cisplatin (IV) prodrug-tethered CuFeS2 nanoparticles in tumor-targeted chemotherapy and photothermal therapy. ACS Appl. Mater. Interfaces 2018, 10, 4590–4602. [Google Scholar] [CrossRef]
- Ma, Y.; Boese, S.E.; Luo, Z.; Nitin, N.; Gill, H.S. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: Development and in vitro evaluation. Biomed. Microdevices 2015, 17, 44. [Google Scholar] [CrossRef]
- Lan, X.; She, J.; Lin, D.-A.; Xu, Y.; Li, X.; Yang, W.-F.; Lui, V.W.Y.; Jin, L.; Xie, X.; Su, Y.-X. Microneedle-Mediated Delivery of Lipid-Coated Cisplatin Nanoparticles for Efficient and Safe Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 33060–33069. [Google Scholar] [CrossRef] [PubMed]
- Romani, N.; Flacher, V.; Tripp, C.; Sparber, F.; Ebner, S.; Stoitzner, P. Targeting skin dendritic cells to improve intradermal vaccination. In Intradermal Immunization; Springer: Berlin/Heidelberg, Germany, 2011; pp. 113–138. [Google Scholar]
- Bol, K.F.; Aarntzen, E.H.; Pots, J.M.; Nordkamp, M.A.O.; van de Rakt, M.W.; Scharenborg, N.M.; de Boer, A.J.; van Oorschot, T.G.; Croockewit, S.A.; Blokx, W.A. Prophylactic vaccines are potent activators of monocyte-derived dendritic cells and drive effective anti-tumor responses in melanoma patients at the cost of toxicity. Cancer Immunol. Immunother. 2016, 65, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, F.; Tötterman, T.; Maltais, A.-K.; Pisa, P.; Yachnin, J. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine 2013, 31, 3843–3848. [Google Scholar] [CrossRef] [PubMed]
- Zaric, M.; Lyubomska, O.; Touzelet, O.; Poux, C.; Al-Zahrani, S.; Fay, F.; Wallace, L.; Terhorst, D.; Malissen, B.; Henri, S. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D, L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano 2013, 7, 2042–2055. [Google Scholar] [CrossRef]
- Kumar, A.; Wonganan, P.; Sandoval, M.A.; Li, X.; Zhu, S.; Cui, Z. Microneedle-mediated transcutaneous immunization with plasmid DNA coated on cationic PLGA nanoparticles. J. Control. Release 2012, 163, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Xu, B.; Xu, J.; Shou, D.; Liu, E.; Gao, J.; Liang, W.; Huang, Y. Microneedle-assisted dendritic cell-targeted nanoparticles for transcutaneous DNA immunization. Polym. Chem. 2015, 6, 373–379. [Google Scholar] [CrossRef]
- Liao, J.; Li, W.; Peng, J.; Yang, Q.; Li, H.; Wei, Y.; Zhang, X.; Qian, Z. Combined cancer photothermal-chemotherapy based on doxorubicin/gold nanorod-loaded polymersomes. Theranostics 2015, 5, 345. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-C.; Lin, Z.-W.; Ling, M.-H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano 2015, 10, 93–101. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; Lei, M.; Zhang, T.; Cao, Y.; Peng, J.; Chen, L.; Qian, Z. Near-Infrared Responsive PEGylated Gold Nanorod and Doxorubicin Loaded Dissolvable Hyaluronic Acid Microneedles for Human Epidermoid Cancer Therapy. Adv. Ther. 2018, 1, 1800008. [Google Scholar] [CrossRef]
- Pei, P.; Yang, F.; Liu, J.; Hu, H.; Du, X.; Hanagata, N.; Zhao, S.; Zhu, Y. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater. Sci. 2018, 6, 1414–1423. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Poly (vinyl alcohol)/chitosan layer-by-layer microneedles for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 576, 118907. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Quan, G.; Wen, T.; Yang, P.; Qin, W.; Mai, H.; Sun, Y.; Lu, C.; Pan, X.; Wu, C. Cold to Hot: Binary Cooperative Microneedle Array Amplified Photo-Immunotherapy for Eliciting Antitumor Immunity and Abscopal Effect. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.J.; McCarron, P.A.; Woolfson, A.D.; Morrissey, A.; Juzenas, P.; Juzeniene, A.; Iani, V.; McCarthy, H.O.; Moan, J. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: Potential for enhanced topical photodynamic therapy. J. Control. Release 2008, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Szeimiesa, R.; Karrera, S.; Radakovic-Fijanb, S.; Tanewb, A.; Calzavara-Pintonc, P.; Zanec, C.; Sidoroffd, A.; Hempele, M.; Ulrichf, J.; Proebstleg, T. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: A prospective, randomized study. J. Am. Acad. Dermatol. 2002, 47, 258–262. [Google Scholar] [CrossRef]
- Morrow, D.I.J.; McCarron, P.A.; Woolfson, A.D.; Juzenas, P.; Juzeniene, A.; Iani, V.; Moan, J.; Donnelly, R.F. Influence of penetration enhancers on topical delivery of 5-aminolevulinic acid from bioadhesive patches. J. Pharm. Pharmacol. 2010, 62, 685–695. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Al-Suwayeh, S.A.; Hung, C.-F.; Chen, C.-C.; Fang, J.-Y. Oil components modulate the skin delivery of 5-aminolevulinic acid and its ester prodrug from oil-in-water and water-in-oil nanoemulsions. Int. J. Nanomed. 2011, 6, 693. [Google Scholar]
- Krishnan, G.; Grice, J.E.; Roberts, M.S.; Benson, H.A.E.; Prow, T.W. Enhanced sonophoretic delivery of 5-aminolevulinic acid: Preliminary human ex vivo permeation data. Skin Res. Technol. 2013, 19, e283–e289. [Google Scholar] [CrossRef]
- Fallows, S.J.; Garland, M.J.; Cassidy, C.M.; Tunney, M.M.; Singh, T.R.R.; Donnelly, R.F. Electrically-responsive anti-adherent hydrogels for photodynamic antimicrobial chemotherapy. J. Photochem. Photobiol. B 2012, 114, 61–72. [Google Scholar] [CrossRef]
- Moothanchery, M.; Seeni, R.Z.; Xu, C.; Pramanik, M. In vivo studies of transdermal nanoparticle delivery with microneedles using photoacoustic microscopy. Biomed. Opt. Express 2017, 8, 5483–5492. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.K.; Lee, C.H.; Gill, H.S. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J. Control. Release 2016, 239, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Tham, H.P.; Chen, H.; Tan, Y.H.; Qu, Q.; Sreejith, S.; Zhao, L.; Venkatraman, S.S.; Zhao, Y. Photosensitizer anchored gold nanorods for targeted combinational photothermal and photodynamic therapy. Chem. Commun. 2016, 52, 8854–8857. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, G.; Zhang, L.; Liu, Z.; Cheng, Z.; Zhu, X. Photosensitizer cross-linked nano-micelle platform for multimodal imaging guided synergistic photothermal/photodynamic therapy. Nanoscale 2016, 8, 15323–15339. [Google Scholar] [CrossRef] [PubMed]
- Tham, H.P.; Xu, K.; Lim, W.Q.; Chen, H.; Zheng, M.; Thng, T.G.S.; Venkatraman, S.S.; Xu, C.; Zhao, Y. Microneedle-Assisted Topical Delivery of Photodynamically Active Mesoporous Formulation for Combination Therapy of Deep-Seated Melanoma. ACS Nano 2018, 12, 11936–11948. [Google Scholar] [CrossRef] [PubMed]
- MacCormack, M.A. Photodynamic therapy. Adv. Dermatol. 2006, 22, 219–258. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.; Venturini, M.; Sala, R. Photodynamic therapy: Update 2006 Part 1: Photochemistry and photobiology. Eur. Acad. Dermatol. Venereol. 2007, 21, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-X.; Ma, M.; Xue, F.; Shen, S.; Chen, Q.; Kuang, Y.; Liang, K.; Wang, X.; Chen, H. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J. Control. Release 2020, 324, 218–227. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef]
- Mistilis, M.J.; Joyce, J.C.; Esser, E.S.; Skountzou, I.; Compans, R.W.; Bommarius, A.S.; Prausnitz, M.R. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv. Transl. Res. 2017, 7, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Mönkäre, J.; Reza Nejadnik, M.; Baccouche, K.; Romeijn, S.; Jiskoot, W.; Bouwstra, J.A. IgG-loaded hyaluronan-based dissolving microneedles for intradermal protein delivery. J. Control. Release 2015, 218, 53–62. [Google Scholar] [CrossRef]
- Seong, K.-Y.; Seo, M.-S.; Hwang, D.Y.; O’Cearbhaill, E.D.; Sreenan, S.; Karp, J.M.; Yang, S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release 2017, 265, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, S.F.; Jang, Y.; Huh, I.; Yang, H.; Jang, M.; Jung, H. Exendin-4–encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci. Rep. 2018, 8, 1170. [Google Scholar] [CrossRef] [Green Version]
- Caudill, C.L.; Perry, J.L.; Tian, S.; Luft, J.C.; DeSimone, J.M. Spatially controlled coating of continuous liquid Interface production microneedles for transdermal protein delivery. J. Control. Release 2018, 284, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, D.D.; Chen, B.Z.; Ashfaq, M.; Guo, X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018, 127, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Rejinold, N.S.; Kwak, J.-E.; Park, S.-H.; Kim, Y.-C. Nano-patterning of a stainless steel microneedle surface to improve the dip-coating efficiency of a DNA vaccine and its immune response. Colloids Surf. B Biointerfaces 2017, 159, 54–61. [Google Scholar] [CrossRef]
- Liu, D.; Yu, B.; Jiang, G.; Yu, W.; Zhang, Y.; Xu, B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng. C 2018, 90, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A. Core–Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, B.; Zhu, J.; Zhang, Y.; Liu, T.; Song, G. Polymer microneedles integrated with glucose-responsive mesoporous bioactive glass nanoparticles for transdermal delivery of insulin. Biomed. Phys. Eng. Express 2019, 5, 045038. [Google Scholar] [CrossRef]
- Tong, Z.; Zhou, J.; Zhong, J.; Tang, Q.; Lei, Z.; Luo, H.; Ma, P.; Liu, X. Glucose- and H2O2-Responsive Polymeric Vesicles Integrated with Microneedle Patches for Glucose-Sensitive Transcutaneous Delivery of Insulin in Diabetic Rats. ACS Appl. Mater. Interfaces 2018, 10, 20014–20024. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Zhang, Y.; Zhou, J.; Sun, S.; Liu, Y. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J. Mater. Chem. B 2017, 5, 8200–8208. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Qian, C.; Lu, Y.; Kahkoska, A.R.; Xie, Z.; Jing, X.; Buse, J.B.; Gu, Z. H2O2-Responsive Vesicles Integrated with Transcutaneous Patches for Glucose-Mediated Insulin Delivery. ACS Nano 2017, 11, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Pang, J.; Wu, X.; Wu, W.; Chen, X.; Kong, M. Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-PD1 immunotherapy via nanovaccine complexed microneedle. Nano Res. 2020, 13, 1509–1518. [Google Scholar] [CrossRef]
- Dul, M.; Nikolic, T.; Stefanidou, M.; McAteer, M.; Williams, P.; Mous, J.; Roep, B.; Kochba, E.; Levin, Y.; Peakman, M. Conjugation of a peptide autoantigen to gold nanoparticles for intradermally administered antigen specific immunotherapy. Int. J. Pharm. 2019, 562, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, N.; Goel, K.; Guo, Y.; McFall, H.; Pillai, A.R.; Shukla, A.; Repka, M.; Murthy, S.N. Stability of Vaccines. AAPS PharmSciTech 2019, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.M.; Cordeiro, A.S.; Kissenpfennig, A.; Donnelly, R.F. Microneedle arrays for vaccine delivery: The possibilities, challenges and use of nanoparticles as a combinatorial approach for enhanced vaccine immunogenicity. Expert Opin. Drug Deliv. 2018, 15, 851–867. [Google Scholar] [CrossRef] [Green Version]
- Suh, H.; Shin, J.; Kim, Y.-C. Microneedle patches for vaccine delivery. Clin. Exp. Vaccine Res. 2014, 3, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.Y.; Ye, L.; Dong, K.; Compans, R.W.; Yang, C.; Prausnitz, M.R. Enhanced stability of inactivated influenza vaccine encapsulated in dissolving microneedle patches. Pharm. Res. 2016, 33, 868–878. [Google Scholar] [CrossRef]
- Rodgers, A.M.; Cordeiro, A.S.; Donnelly, R.F. Technology update: Dissolvable microneedle patches for vaccine delivery. Med. Devices 2019, 12, 379. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Sun, J.; Zhuang, J.; Xu, H.; Liu, Y.; Wu, D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects. Dose-Response 2019, 17, 1559325819878585. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Du, G.; Hathout, R.M.; Nasr, M.; Nejadnik, M.R.; Tu, J.; Koning, R.I.; Koster, A.J.; Slütter, B.; Kros, A.; Jiskoot, W.; et al. Intradermal vaccination with hollow microneedles: A comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J. Control. Release 2017, 266, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Haigh, O.; Depelsenaire, A.C.I.; Meliga, S.C.; Yukiko, S.R.; McMillan, N.A.J.; Frazer, I.H.; Kendall, M.A.F. CXCL1 gene silencing in skin using liposome-encapsulated siRNA delivered by microprojection array. J. Control. Release 2014, 194, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. 2017, 13, 921–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Kim, H.; Park, Y.; Kong, W.H.; Lee, S.W.; Kwok, S.J.J.; Hahn, S.K.; Yun, S.H. Noninvasive Transdermal Vaccination Using Hyaluronan Nanocarriers and Laser Adjuvant. Adv. Funct. Mater. 2016, 26, 2512–2522. [Google Scholar] [CrossRef]
- Siddhapura, K.; Harde, H.; Jain, S. Immunostimulatory effect of tetanus toxoid loaded chitosan nanoparticles following microneedles assisted immunization. Nanomed. Nanotechnol. 2016, 12, 213–222. [Google Scholar] [CrossRef]
- Yin, D.; Liang, W.; Xing, S.; Gao, Z.; Zhang, W.; Guo, Z.; Gao, S. Hepatitis B DNA Vaccine-Polycation Nano-Complexes Enhancing Immune Response by Percutaneous Administration with Microneedle. Biol. Pharm. Bull. 2013, 36, 1283–1291. [Google Scholar] [CrossRef] [Green Version]
- Caucheteux, S.M.; Mitchell, J.P.; Ivory, M.O.; Hirosue, S.; Hakobyan, S.; Dolton, G.; Ladell, K.; Miners, K.; Price, D.A.; Kan-Mitchell, J.; et al. Polypropylene Sulfide Nanoparticle p24 Vaccine Promotes Dendritic Cell-Mediated Specific Immune Responses against HIV-1. J. Investig. Dermatol. 2016, 136, 1172–1181. [Google Scholar] [CrossRef] [Green Version]
- Seok, H.; Noh, J.Y.; Lee, D.Y.; Kim, S.J.; Song, C.S.; Kim, Y.C. Effective humoral immune response from a H1N1 DNA vaccine delivered to the skin by microneedles coated with PLGA-based cationic nanoparticles. J. Control. Release 2017, 265, 66–74. [Google Scholar] [CrossRef]
- Pearton, M.; Pirri, D.; Kang, S.-M.; Compans, R.W.; Birchall, J.C. Host Responses in Human Skin After Conventional Intradermal Injection or Microneedle Administration of Virus-Like-Particle Influenza Vaccine. Adv. Healthc. Mater. 2013, 2, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- de Groot, A.M.; Du, G.; Mönkäre, J.; Platteel, A.C.M.; Broere, F.; Bouwstra, J.A.; Sijts, A.J.A.M. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium. J. Control. Release 2017, 266, 27–35. [Google Scholar] [CrossRef]
- Pamornpathomkul, B.; Niyomtham, N.; Yingyongnarongkul, B.-E.; Prasitpuriprecha, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Cationic Niosomes for Enhanced Skin Immunization of Plasmid DNA-Encoding Ovalbumin via Hollow Microneedles. AAPS PharmSciTech 2018, 19, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Leone, M.; Romeijn, S.; Kersten, G.; Jiskoot, W.; Bouwstra, J.A. Immunogenicity of diphtheria toxoid and poly(I:C) loaded cationic liposomes after hollow microneedle-mediated intradermal injection in mice. Int. J. Pharm. 2018, 547, 250–257. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Qiu, Y.; Zhang, S.; Xu, B.; Gao, Y. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int. J. Pharm. 2013, 447, 22–30. [Google Scholar] [CrossRef]
- Zhen, Y.; Wang, N.; Gao, Z.; Ma, X.; Wei, B.; Deng, Y.; Wang, T. Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine 2015, 33, 4330–4340. [Google Scholar] [CrossRef]
- Cole, G.; McCaffrey, J.; Ali, A.A.; McBride, J.W.; McCrudden, C.M.; Vincente-Perez, E.M.; Donnelly, R.F.; McCarthy, H.O. Dissolving microneedles for DNA vaccination: Improving functionality via polymer characterization and RALA complexation. Hum. Vaccines Immunother. 2017, 13, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.-H.; Zhang, Q.-B.; Liu, B.; Piao, X.-H.; Yan, Y.-L.; Hu, X.-G.; Zhou, K.; Zhang, Y.-T.; Feng, N.-P. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int. J. Nanomed. 2017, 12, 4763–4772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Li, Y.; Chen, X.; Zhou, Z.; Pang, J.; Luo, X.; Kong, M. A surface charge dependent enhanced Th1 antigen-specific immune response in lymph nodes by transfersome-based nanovaccine-loaded dissolving microneedle-assisted transdermal immunization. J. Mater. Chem. B 2019, 7, 4854–4866. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Nguyen, T.L.; Giang Phan, V.H.; Lee, M.S.; Lee, J.E.; Kim, J.; Jeong, J.H.; Lee, D.S. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials 2018, 185, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Rattanapak, T.; Birchall, J.; Young, K.; Ishii, M.; Meglinski, I.; Rades, T.; Hook, S. Transcutaneous immunization using microneedles and cubosomes: Mechanistic investigations using Optical Coherence Tomography and Two-Photon Microscopy. J. Control. Release 2013, 172, 894–903. [Google Scholar] [CrossRef]
- Mulligan, R.C. The basic science of gene therapy. Science 1993, 260, 926–932. [Google Scholar] [CrossRef]
- Chen, X. Current and future technological advances in transdermal gene delivery. Adv. Drug Deliv. Rev. 2018, 127, 85–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.W.; Lee, M.S.; Kim, K.R.; Lee, J.E.; Lee, K.; Park, J.S.; Matsumoto, Y.; Jo, D.-G.; Lee, H.; Lee, D.S. Polyplex-releasing microneedles for enhanced cutaneous delivery of DNA vaccine. J. Control. Release 2014, 179, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Zhai, Y.; Yu, K.; Wu, C.; Xu, Y. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int. J. Pharm. 2018, 553, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, B.; Tao, J.; Yang, Y.; Hu, Y.; Huang, Y. Microneedle-Assisted, DC-Targeted Codelivery of pTRP-2 and Adjuvant of Paclitaxel for Transcutaneous Immunotherapy. Small 2017, 13, 1700666. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Wheeler, D.C.S.; Peakman, M. Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet Diabetes Endocrinol. 2019, 7, 65–74. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 31 December 2020).
- Ogunjimi, A.T.; Carr, J.; Lawson, C.; Ferguson, N.; Brogden, N.K. Micropore closure time is longer following microneedle application to skin of color. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]







| Type of MNs | Advantages | Drawbacks | Applications |
|---|---|---|---|
| Solid | Technically simple, without any pump or loading/coating procedure, small doses can be administered | Two-step administration procedure, no exact dosing, drugs need to be reformulated | Skin pretreatment for the delivery of insulin [55], cosmetics [56], vaccines [57], potassium chloride [58], monitoring of lactate and glucose [59], urea sensing [60] |
| Coated | MN strength is retained after coating, without any patch or pump, precise dosing is possible | Appropriate coating technique is needed, limited to small doses, drugs need to be reformulated, coatings might lessen MN sharpness | Delivery of vaccines [61], insulin [62], proteins [63], desmopressin [64], parathyroid hormone [65], sampling, isolation and identification of biomarkers [66] |
| Hollow | Drug delivery rates can be controlled, delivery of a relatively high liquid volume is plausible, possible combination with lab-on-a-chip devices, precise dosing | Possible risk of clogging, reduced MN strength, possible risk of drug leakage, complex devices | Delivery of vaccines [67], insulin [68], cell therapy [69], delivery of mRNA [70], DNA (pDNA) [71], biofluid extraction and bio-signal detection [72,73], colorimetric detection of glucose [74] |
| Dissolving | No need for any pump or patch, precise dosing is possible, no sharp waste, low preparation costs | Small drug doses may be lost throughout the encapsulation/absorption procedure, low strength, low penetration ability, limited to small drug doses, drug reformulation is needed | Delivery of vaccines [75,76], insulin [77], therapeutic peptides [78], triamcinolone acetonide [79], doxorubicin [80], epidermal growth factor and ascorbic acid [81], adenosine [82], vitamin B12 [83], near-IR photosensitizer (Redaporfin™) [84], sodium nitroprusside in combination with sodium thiosulfate [85], DNA extraction [86] |
| Hydrogel-forming | No need for any pump or patch, precise dosing is possible, no sharp waste | Small drug doses may be lost throughout the encapsulation/absorption procedure, limited to small drug doses, low strength, and penetration ability, drug reformulation is needed | Delivery of vaccines [87], metformin hydrochloride [50], methotrexate [53], caffeine [46]. glucose-responsive insulin delivery [51], stimulus-responsive ibuprofen delivery [47], lithium monitoring [88], theophylline, caffeine and glucose monitoring [89] |
| Type of MNs | Carrier | Drug | Outcome | Ref. |
|---|---|---|---|---|
| Solid (stainless steel) | Microemulsions | Propranolol | Enhanced permeation | [103] |
| Solid (silicon) | Dextran NPs | Rosiglitazone CL 316243 | Localized and painless administration in a safe and effective manner | [104] |
| Solid (silicon) | Microemulsion | Tetramethyl pyrazine | Enhanced percutaneous absorption of drug-loaded microemulsions by MNs | [68] |
| Solid (rolling MNs) | Ethosomes | Paeoniflorin | Enhanced skin penetration, no synergistic effect in combination of MNs with ethosomes | [101] |
| Solid (not defined) | NLC | Alkaloids | Enhanced skin penetration with combination of MNs and NLCs, increased transdermal bioavailability of alkaloids, consistent blood drug concentrations | [105] |
| Solid (stainless steel) | Polymeric NPs (carboxymethyl chitosan) | 5-Fuorouracil | Localized and painless administration, low side effects | [106] |
| Dissolving (PVP) | PLGA hollow microspheres | Alexa 488 and Cy5 (model compounds) | Co-delivery into the skin | [43] |
| Dissolving (PEG-MGQD) | nanocomposites of chitosan and magnetic graphene quantum dot | Lidocaine hydrochloride | Delivery of small and large molecule therapeutics | [107] |
| Dissolving (PVP) | PLGA nano/microparticles | Vitamin D3 | Delivery to deep skin layers and sustained release | [108] |
| Dissolving (PVP) | Hollow mesoporous silica nanocomposites | Metformin | NIR-triggered TDD and photothermal-responsive delivery | [109] |
| Dissolving (PVP) | PLGA@chitosan and PCL@chitosan NPs | Doxycycline | Enhanced retention and improved dermatokinetic profile of doxycycline | [110] |
| Dissolving (PVP) | Nanosuspension | Doxycycline, Albendazole, and Ivermectin | Enhance retention in the dermis | [111] |
| Dissolving (PVA/PVP) | PCL NPs | Carvacrol | Enhanced skin retention, sustained therapeutic effect | [112] |
| Dissolving (HA) | HA NPs | Rhodamine B | Enhanced skin penetration, sustained release | [113] |
| Dissolving (PVA) | Nanosuspension | Curcumin | Improved intradermal delivery | [114] |
| Dissolving (HA) | MPEG-PCL NPs | 5-Fuorouracil and indocyanine green | NIR-responsive delivery, synergistic chemo-photothermal effect | [115] |
| Dissolving (HA) | Micelles | Curcumin | Enhanced transdermal permeation | [116] |
| Dissolving (PVA/PVP) | SLNs | Doxycycline, Diethylcarbamazine, and Albendazole | Enhanced retention in the dermis layer, high bioavailability, accumulation in lymph nodes | [117] |
| Dissolving (PVA) | Micelles | Rhodamine B | TDD of kidney targeting NPs | [118] |
| Hydrogel-forming (PLGA) | Hydrogel microparticles | Rhodamine B | Sustained release | [119] |
| Type of MNs | Vaccine Type | Carrier | Outcome | Ref. |
|---|---|---|---|---|
| Solid | Fluvax vaccine + CXCL1-specific siRNA | Liposome | Effective silencing of CXCL1 gene in skin | [174] |
| Solid | HPV-16 E6/E7 DNA | PVP | More potent vaccination than intramuscular administration, delayed TC-1 tumor growth | [175] |
| Solid | Ovalbumin | Hyaluronan | Humoral and mucosal immune activation, strong immune-recall responses, strong immunization | [176] |
| Solid | Tetanus toxoid | Chitosan | Higher IgG2a level in comparison to commercial vaccine, balanced Th1/Th2 ratio | [177,178] |
| Coated | HIV-1 p24 Gag peptide | Polypropylene sulfide | Efficient uptake without adjuvant, potent HIV-1specific CD4+ T-cell responses | [179] |
| Coated | pH1N1 DNA | Polyplex containing PLGA/poly ethyleneimine (PEI) | Rapid dissolution of polyplex which induced potent humoral immune response | [180] |
| Hollow | H1N1 virus | Virus like nanoparticles (VLPs) | New vaccination approach | [181] |
| Hollow | Ovalbumin | PLGA | Protective T cell-mediated immunity | [182] |
| Hollow | Plasmid DNA | Niosome | High level of IgG titer | [183] |
| Hollow | Diphtheria toxoid | Liposome | High level of IgG2a | [184] |
| Hollow | Peptide/ Human papillomavirus | Liposome | Strong T helper responses | [184] |
| Dissolving | Ovalbumin | Liposome | High IgG level, enhanced immune response | [185] |
| Dissolving | Ovalbumin | Liposome | Effective mucosal immunization via oral route vaccination | [186] |
| Dissolving | pEGFP-N1 plasmid DNA | PVP and PVA | Superior DNA preservation by PVA than PVP | [187] |
| Dissolving | Ovalbumin | Liposome | Enhanced immune response, balanced Th1 and Th2 humoral immune responses | [188] |
| Dissolving | Ovalbumin | Transfersomes | Increased IgG2a/IgG1 ratio, specific Th1 antigen-specific immunizations Immune response in lymph nodes | [189] |
| Dissolving | Hepatitis B virus | Liposome | Strong cell-mediated immune response HBV, CD8+ T cells population increase significantly | [186] |
| Dissolving | Ovalbumin Plasmid | OSM-(PEG-PAEU) * | Humoral and cellular immunity, activation of cytotoxic CD8+ T cells | [190] |
| Dissolving | DNA | Pluronic P123/PEI | Higher humoral and cellular immunity compared to IM administration | [177,178] |
| Dissolving | SIINFEKL peptide | Cubosome | Efficient local delivery. | [191] |
| Field | Title | Type of MNs | Condition/Disease | Phase | Status | Clinical Trial Registry Number |
|---|---|---|---|---|---|---|
| Therapeutic | 2010/2011 trivalent influenza vaccination | Solid (MicronJet) | Influenza | Not provided | Completed | NCT01304563 |
| The effect of microneedle pretreatment on topical anesthesia | Solid (MN roller) | Pain | Not provided | Completed | NCT02596750 | |
| The use of microneedles to expedite treatment time in photodynamic therapy | Solid (MN roller) | Keratosis, actinic | Not provided | Completed | NCT02594644 | |
| The use of microneedles with topical botulinum toxin for treatment of palmar hyperhidrosis | Solid (Sham MN) | Hyperhidrosis | I | Completed | NCT03203174 | |
| A study to determine the patient preference between Zosano Pharma parathyroid hormone (ZP-PTH) patch and Forteo pen | Coated titanium (ZP-PTH MN patch) | Postmenopausal Osteoporosis | I | Completed | NCT02478879 | |
| Phase 2 study of BA058 (Abaloparatide) transdermal delivery in postmenopausal women with osteoporosis | Coated 3 M microstructured transdermal system | Post-menopausal osteoporosis | II | Completed | NCT01674621 | |
| Dose sparing intradermal H1N1 influenza vaccination device | Hollow (MicronJet 600™) | Influenza infection | Not provided | Completed | NCT01049490 | |
| Immunogenicity of inactivated and live polio vaccines | Hollow (MicronJet 600™) | Poliomyelitis | III | Unknown | NCT01813604 | |
| Safety study of suprachoroidal triamcinolone acetonide via microneedle to treat uveitis | Hollow | Uveitis | I/II | Completed | NCT01789320 | |
| Routes of immunization and flu immune responses | MN injection (not defined) | Influenza | I/II | Completed | NCT01707602 | |
| Comparison of 4 influenza vaccines in seniors | Hollow (Becton Dickinson (BD) MN) | Influenza | IV | Completed | NCT01368796 | |
| Immunogenicity of the inactivated split-virion influenza vaccine in renal transplant subjects | Hollow (BD Soluvia™) | Influenza Orthomyx-oviridae infection | II | Completed | NCT00606359 | |
| Atopic dermatitis research network (adrn) influenza vaccine study | Pre-filled or hollow (Fluzone®) | Dermatitis, atopic | Not provided | Completed | NCT01737710 | |
| Immunogenicity study of the influenza vaccine in adults | Hollow (BD MN injection) | Orthomyx-oviridae infection Influenza | II | Completed | NCT00258934 | |
| Study of inactivated, split-virion influenza vaccine compared with the reference vaccine Vaxigrip® in the elderly | Hollow (BD MN injection) | Orthomyx-oviridae infection Influenza Myxovirus infection | III | Completed | NCT00383526 | |
| Intradermal versus intramuscular polio vaccine booster in HIV-infected subjects | Hollow (MicronJet 600™) | Polio immunity | II | Completed | NCT01686503 | |
| Varicella zoster virus (VZV) vaccine for hematopoietic stem cell transplantation | Hollow (MN syringe) | Varicella zoster infection | II/III | Completed | NCT02329457 | |
| Insulin delivery using microneedles in type 1 diabetes | Hollow (glass) | Type 1 Diabetes Mellitus | II/III | Completed | NCT00837512 | |
| A pilot study to assess the safety, pharmacokinetics/ pharmacodynamics (PK/PD) of insulin injected via MicronJet™ or conventional needle | Hollow (MicronJet™) | Intradermal injections | Early phase I | Completed | NCT00602914 | |
| Pharmacokinetic comparison of intradermal versus sub-cutaneous insulin and glucagon delivery in type 1 diabetes | Hollow (MicronJet™) | Type 1 Diabetes | II | Unknown | NCT01684956 | |
| Multi-day (3) in-patient evaluation of intradermal versus subcutaneous basal and bolus insulin infusion | Hollow (BD Research Catheter) | Diabetes | I/II | Completed | NCT01557907 | |
| Safety and efficacy of ZP-glucagon to injectable glucagon for hypoglycemia | Not defined | Hypoglycemia | I | Completed | NCT02459938 | |
| Study on the effects on blood glucose following intradermal and subcutaneous dosing of insulin in diabetic patients | Hollow (BD Research Catheter) | Diabetes | I/II | Completed | NCT01120444 | |
| Pharmacokinetics/dynamics of basal (continuous) insulin infusion administered either intradermally or subcutaneously | Hollow (BD Research Catheter) | Diabetes Mellitus, Type 1/2 | I/II | Completed | NCT01061216 | |
| A study to assess the safety and efficacy of a microneedle device for local anesthesia | Hollow (MicronJet™) | Local anesthesia intradermal injections | Not provided | Completed | NCT00539084 | |
| Safety demonstration of microneedle insertion | Gold/silver coated or uncoated hollow MNs | Allergic reaction to nickel | NA (safety demonstration) | Completed | NCT02995057 | |
| Microneedle patch study in healthy infants/young children | Dissolvable | Vaccination skin absorption | Not provided | Completed | NCT03207763 | |
| Microneedle array–doxorubicin (MNA-D) in patients with cutaneous T-cell lymphoma (CTCL) | Dissolvable | Cutaneous T cell lymphoma | I | Recruiting | NCT02192021 | |
| Inactivated influenza vaccine delivered by microneedle patch or by hypodermic needle | Dissolvable | Influenza | I | Completed | NCT02438423 | |
| Microneedle patch for psoriatic plaques | Dissolvable (MN-HA patch) | Psoriasis | Not provided | Unknown | NCT02955576 | |
| Safety and efficacy of ZP-zolmitriptan intracutaneous microneedle systems for the acute treatment of migraine | MN patch (not defined) | Acute migraine | II/III | Completed | NCT02745392 | |
| Cosmetic | Microneedling plus the universal peel for acne scarring | Solid (MicroPen) | Acne scarring | Not provided | Completed | NCT02174393 |
| Comparison of efficacy between fractional microneedling radiofrequency and bipolar radiofrequency for acne scar | Solid (Microneedling radiofrequency device) | Acne scarring | Not provided | Completed | NCT02207738 | |
| Comparison of treatments for atrophic acne scars | Solid (Dermaroller) | Acne scarring | Not provided | Unknown | NCT02025088 | |
| Comparison of the efficacy of micro-holes versus laser-assisted dermabrasion, for repigmenting in vitiligo skin | Solid (Dermaroller) | Vitiligo-macular depigmentation | Not provided | Unknown | NCT02660320 | |
| Transplantation of Basal Cell Layer Suspension Using Derma-rolling System in Vitiligo | Solid (Dermaroller) | Vitiligo | Not provided | Unknown | NCT02962180 | |
| Safety and efficacy of the EndyMed Pro system using RF microneedles fractional skin remodeling | Solid (EndyMed Pro™ RF Microneedles) | Aging | Not provided | Unknown | NCT02368626 | |
| Evaluating the efficacy of microneedling in the treatment of androgenetic alopecia | Solid (Microneedling) | Androgenetic alopecia | I | Unknown | NCT02154503 | |
| Performance of the ePrime System for Cellulite | Solid (ePrime Syneron Candela) | Cellulite | Not provided | Unknown | NCT02489994 | |
| Tolerability study of the application of a 3M microstructure transdermal system | Solid (Transdermal Microchannel Skin System) | Healthy | I | Completed | NCT01257763 | |
| Teosyal® PureSense redensity [I] injection using Micronjet® needle in the treatment of crow’s feet wrinkles | Hollow (MicronJet™) | Crow’s feet wrinkles | IV | Completed | NCT02497846 | |
| Diagnostic | Physiological study to determine the allergic skin activity after different skin preparation | Solid (Micro Skin System, 3M) | Birch pollen allergy | I | Completed | NCT01628484 |
| Minimally Invasive Sensing of Beta-lactam Antibiotics (MISBL) | Solid | Healthy volunteers | I | Completed | NCT03847610 | |
| Analysis of non-invasively collected microneedle device samples from mild plaque psoriasis for use in transcriptomics profiling | Solid MNs as sampling device | Psoriasis vulgaris | Cohort, prospective | Completed | NCT03795402 | |
| Optimization of tuberculosis intradermal skin test | Hollow (BD Research Catheter) | Healthy volunteers | Not provided | Completed | NCT01611844 | |
| Glucose measurement using microneedle patches | Hydrogel forming | Diabetes (diagnostic) | Not provided | Completed | NCT02682056 |
| Type of MNs | Product Name | Company Name | Description of the Product | Use |
|---|---|---|---|---|
| Solid | Dermapen | Dermaroller GmbH | An array of 12 needles loaded on an electric motor unit fitted with a spring that punches skin (412–700 cycle/min) | Treatment of isolated scars, skin lesions and wrinkles, appropriate for smaller areas of the skin |
| Dermaroller | Whitelotusbeauty | Contains 192 titanium needles (0.5 mm long) in cylindrical assembly | Cosmetic application and skin care with cream and serum, appropriate for larger areas of the skin | |
| Dermastamp | Whitelotusbeauty | An array of 40 needles loaded on an electric motor unit with controlled motion back and forth like stamp | Collagen induction therapy for skin scars, age spots, varicella scars and wrinkles, appropriate for smaller areas of the skin | |
| DermaFrac | Dermafrac.co | Very small stainless steel MNs roller equipped with electric power source and instrument for serum infusion, also contains light emitting devices | Wrinkles, skin ageing, hyperpigmentation, acne, uneven skin tone | |
| Onvax | Becton Dickinson | An array of silicon or plastic micro-projections on a hand-held applicator | Vaccine delivery | |
| LiteClear | Nanomed skincare | Silicon MNs pen for skin pretreatment | Treatment of acne and skin blemishes | |
| h-patch | Valeritas | Small adhesive MNs, hydrolytically regulated | Basal and bolus delivery of insulin | |
| Beauty Mouse | Dermaroller GmbH | Three rollers of 50 mm width and a total of 480 MNs, creating fine microchannels for enhanced penetration | Increasing the skin’s sensitivity towards anti-cellulite creams | |
| NanoCare | NanoPass Inc. | A small hand-held device for the rejuvenation of skin and to boost the cosmetic effect of topical applications | Cosmetic | |
| Adminstamp | AdminMed | MNs array attached to the applicator with six stainless steel screws (1400 µm-long MNs on 1 cm2 circular array), compatible with all sterilization methods | Transdermal drug delivery through the skin with excellent skin sensation and cosmetics | |
| Coated | MacroFLUX™ | Zosano pharma | PTH-coated titanium MN patch | Osteoporosis |
| Nanopatch | Vaxxas | Silicon patch (1 cm2) made up thousands of coated micro-projections | Polio vaccine | |
| Hollow | 3M MTS | 3M Corp | MN patch containing 351 needles/cm2 (650 µm length) | Skin treatment before dermatological application |
| FLUARIX | GlaxoSmithKline Biologicals | Three MNs (600 µm length) attached to a syringe | Influenza vaccine delivery | |
| Micronjet | Nanopass | Four silicon MNs (450µm length) attached to the tip of a plastic adapter | Intradermal vaccine delivery | |
| Fluzone | Sanofi Pasteur Inc Becton Dickinson | A hand syringe gun unit that injects 1.5 mL vaccine solution by a micro-injector in intradermal site by a 1.5 mm MN tip | Influenza vaccine | |
| Soluvia | Sanofi-Aventis Becton Dickinson | MNs (length 1.5 mm) for intradermal delivery | Influenza vaccine | |
| Microinfusor | Becton Dickinson | An automated hands-free system consists of an electrical pump connected to a hollow MN patch (capacity 0.2–15 mL) | Influenza vaccine, insulin, and highly viscose biotech drug | |
| Nanoject | Debiotech | Based on MEMS technology, hollow MN patch (length 300 to 1000 µm), featuring side holes which prevent sticking in the MN channels during skin penetration | Intradermal drug delivery and injection of diagnostic fluid | |
| Micro-Trans | Valeritas Inc. | Hollow MNs constructed with metal or biodegradable polymers | Intradermal drug delivery | |
| Intanza/IDflu | Sanofi-Aventis Becton Dickinson | MNs (length 1.5 mm) combined with a needle shielding system and a 0.1 mL injection volume | Influenza vaccine | |
| AdminPen | AdminMed | Forty-three needles (length 1100 µm) on 1 cm2 circular microneedle array, stainless steel liquid injector device attached to a standard syringe | Liquid formulations (vaccines, drug) | |
| Microinject | Nanopass | Four hollow silicon MNs (length 250 µm), fitted with a syringe for intradermal injection | Influenza vaccine | |
| DebioJect | Debiotech | One or several hollow silicon MNs with a length ranging from 350 to 900 µm and a side protected delivery holes, injections up to 0.5 mL in a few seconds | Vaccine delivery | |
| Dissolving | Drugmat® | Theraject Inc. | Sumatriptan-loaded dMN patch made from a sugar polysaccharide | Migraine |
| Vaxmat® | Theraject Inc. | Sumatriptan-load dMNs | Migraine | |
| MicroCor | Coriumintl | PTH-loaded dissolvable peptide MN patch | Osteoporosis | |
| MicroHyala | CosMed | MN patch made of biocompatible hyaluronic acid | Wrinkle treatment, influenza vaccine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M.; Tamaddon, A.; Ahadian, S. Microneedle Arrays Combined with Nanomedicine Approaches for Transdermal Delivery of Therapeutics. J. Clin. Med. 2021, 10, 181. https://doi.org/10.3390/jcm10020181
Alimardani V, Abolmaali SS, Yousefi G, Rahiminezhad Z, Abedi M, Tamaddon A, Ahadian S. Microneedle Arrays Combined with Nanomedicine Approaches for Transdermal Delivery of Therapeutics. Journal of Clinical Medicine. 2021; 10(2):181. https://doi.org/10.3390/jcm10020181
Chicago/Turabian StyleAlimardani, Vahid, Samira Sadat Abolmaali, Gholamhossein Yousefi, Zahra Rahiminezhad, Mehdi Abedi, Alimohammad Tamaddon, and Samad Ahadian. 2021. "Microneedle Arrays Combined with Nanomedicine Approaches for Transdermal Delivery of Therapeutics" Journal of Clinical Medicine 10, no. 2: 181. https://doi.org/10.3390/jcm10020181