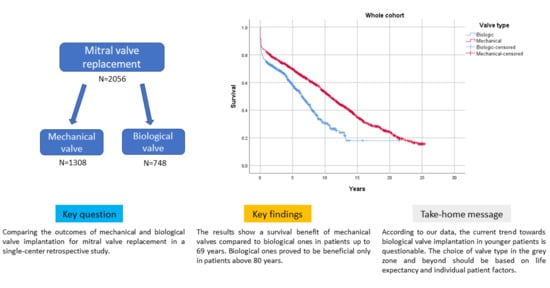
Long-Term Outcomes Stratified by Age in Patients with a Mechanical versus Biological Mitral Valve Replacement
Abstract
:1. Introduction
2. Patients and Methods
2.1. Ethical Statement
2.2. Study Design
2.3. Study Population
2.4. Statistical Analysis
3. Results
3.1. Preoperative and Intraoperative Characteristics
Survival
3.2. Postoperative Characteristics
4. Discussion
4.1. Survival
4.2. Trends in the Application of Mechanical and Biological Valves
4.3. Postoperative Outcomes
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Gummert, J. German Heart Surgery Report 2019: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2020, 68, 263–276. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, J.; Casselman, F. Mitral Valve Replacement—Current and Future Perspectives. Open J. Cardiovasc. Surg. 2017, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- Chikwe, J.; Chiang, Y.P.; Egorova, N.N.; Itagaki, S.; Adams, D.H. Survival and Outcomes Following Bioprosthetic vs. Mechanical Mitral Valve Replacement in Patients Aged 50 to 69 Years. JAMA 2015, 313, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, A.; Poggenpohl, J.; Bramlage, K.; Hein, S.; Doss, M.; Bramlage, P.; Schönburg, M.; Richter, M. Long-term outcome after mitral valve replacement using biological versus mechanical valves. J. Cardiothorac. Surg. 2019, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Schnittman, S.R.; Itagaki, S.; Toyoda, N.; Adams, D.H.; Egorova, N.N.; Chikwe, J. Survival and long-term outcomes after mitral valve replacement in patients aged 18 to 50 years. J. Thorac. Cardiovasc. 2018, 155, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Nishida, T.; Sonoda, H.; Oishi, Y.; Tanoue, Y.; Nakashima, A.; Shiokawa, Y.; Tominaga, R. Mechanical Prosthesis Is Reasonable for Mitral Valve Replacement in Patients Approximately 65 Years of Age. Ann. Thorac. Surg. 2013, 96, 1614–1620. [Google Scholar] [CrossRef]
- Jamieson, W.; von Lipinski, O.; Miyagishima, R.; Burr, L.; Janusz, M.; Ling, H.; Fradet, G.; Chan, F.; Germann, E. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after mitral valve replacement. J. Thorac. Cardiovasc. Surg. 2005, 129, 1301–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulik, A.; Bédard, P.; Lam, B.-K.; Rubens, F.D.; Hendry, P.J.; Masters, R.G.; Mesana, T.G.; Ruel, M. Mechanical versus bioprosthetic valve replacement in middle-aged patients. Eur. J. Cardio-Thorac. Surg. 2006, 30, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruel, M.; Chan, V.; Bédard, P.; Kulik, A.; Ressler, L.; Lam, B.K.; Rubens, F.D.; Goldstein, W.; Hendry, P.J.; Masters, R.G.; et al. Very long-term survival implications of heart valve replacement with tissue versus mechanical prostheses in adults. Circulation 2007, 116, I-294–I-300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.D.; Ad, N. Surgery for aortic and mitral valve disease in the United States: A trend of change in surgical practice between 1998 and 2005. J. Thorac. Cardiovasc. Surg. 2009, 137, 1422–1429. [Google Scholar] [CrossRef] [Green Version]
- Chikwe, J.; Filsoufi, F. Durability of tissue valves. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 18–23. [Google Scholar] [CrossRef]
- Hammermeister, K.; Sethi, G.K.; Henderson, W.G.; Grover, F.L.; Oprian, C.; Rahimtoola, S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: Final report of the Veterans Affairs randomized trial. J. Am. Coll. Cardiol. 2000, 36, 1152–1158. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.S.; Trento, A.; DeRobertis, M.; Kass, R.M.; Sandhu, M.; Czer, L.S.; Blanche, C.; Raissi, S.; Fontana, G.P.; Cheng, W.; et al. Twenty-year comparison of tissue and mechanical valve replacement. J. Thorac. Cardiovasc. Surg. 2001, 122, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of bioprosthetic heart valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef]
- Çelik, M.; Durko, A.P.; Head, S.J. Anticoagulation after mechanical aortic valve implantation: Is it time to act after PROACT? Ann. Transl. Med. 2018, 6, 1–4. [Google Scholar] [CrossRef]
- Melfi, R.; Ricottini, E. Antiplatelet therapy for peripheral artery disease. Cardiovasc. Diagn. Ther. 2018, 8, 663–677. [Google Scholar] [CrossRef] [PubMed]



| Total Cohort | Biological | Mechanical | p Value | |
|---|---|---|---|---|
| Pulmonary stasis | 299/2034 (14.7%) | 123/743 (16.6%) | 176/1291 (13.6%) | 0.073 |
| Liver enlargement | 205/2021 (10.1%) | 49/738 (6.6%) | 156/1283 (12.2%) | <0.001 |
| Peripheral oedema | 519/2037 (25.5%) | 156/744 (21.0%) | 363/1293 (28.1%) | <0.001 |
| Embolic accidents | 135/2032 (6.6%) | 31/747 (4.1%) | 104/1285 (8.1%) | <0.001 |
| Cardiac decompensation | 691/2038 (33.9%) | 267/747 (35.7%) | 424/1291 (32.8%) | 0.183 |
| Stroke | 219/1578 (13.9%) | 107/727 (14.7%) | 112/851 (13.2%) | 0.373 |
| Diabetes mellitus | 484/2033 (23.8%) | 207/747 (27.7%) | 277/1286 (21.5%) | 0.002 |
| Hyperlipoproteinemia | 864/1992 (43.4%) | 374/741 (50.5%) | 490/1251 (39.2%) | <0.001 |
| Hypertension | 1390/2017 (68.9%) | 606/745 (81.3%) | 784/1272 (61.6%) | <0.001 |
| Pulmonary hypertension | 837/1575 (53.1%).7 | 401/726 (55.2%) | 436/849 (51.4%) | 0.124 |
| Peripheral arterial disease | 116/1579 (7.3%) | 58/726 (8.0%) | 58/853 (6.8%) | 0.367 |
| Carotid stenosis | 128/1573 (8.1%) | 65/725 (9.0%) | 63/848 (7.4%) | 0.267 |
| History of smoking | 554/1809 (30.6%) | 190/710 (26.8%) | 364/1099 (33.1%) | 0.004 |
| Etiology | ||||
| Endocarditis | 287/2056 (14.0%) | 126/748 (16.8%) | 161/1308 (12.3%) | 0.004 |
| Stenosis | 208/2056 (10.1%) | 46/748 (6.1%) | 162/1308 (12.4%) | <0.001 |
| Insufficiency | 1390/2056 (67.6%) | 567/748 (75.8%) | 823/1308 (62.9%) | <0.001 |
| Stenosis+ Insufficiency | 458/2056 (22.3%) | 135/748 (18.0%) | 323/1308 (24.7%) | <0.001 |
| Antithrombotic Therapy | ||||
| Aspirin | 466/2012 (23.2%) | 248/740 (33.5%) | 218/1272 (17.1%) | <0.001 |
| Other platelet aggregation inhibitors | 33/1798 (1.8%) | 28/626 (4.5%) | 5/1172 (0.4%) | <0.001 |
| Anticoagulants | 762/2014 (37.8%) | 237/740 (32.0%) | 525/1274 (41.2%) | <0.001 |
| NYHA Classification | ||||
| NYHAI | 53/2053 (2.6%) | 9/746 (1.2%) | 44/1307 (3.4%) | 0.003 |
| NYHAII | 301/2053 (14.7%) | 113/746 (15.1%) | 188/1307 (14.4%) | 0.638 |
| NYHAIII | 1142/2053 (55.6%) | 453/746 (60.7%) | 689/1307 (52.7%) | <0.001 |
| NYHAIV | 557/2053 (27.1%) | 171/746 (22.9%) | 386/1307 (29.5%) | <0.001 |
| Total Cohort | Biological | Mechanical | p Value | |
|---|---|---|---|---|
| Approach | ||||
| Median sternotomy | 1937/2049 (94.5%) | 690/748 (92.2%) | 1247/1301 (95.8%) | <0.001 |
| MIC | 112/2049 (5.5%) | 58/748 (7.8%) | 54/1301 (4.2%) | <0.001 |
| Valve characteristics | ||||
| Size | 31 (29–31) | 29 (29–31) | 31 (29–32) | <0.001 * |
| Types (n) | Carpentier-Edwards Physio II 5200 (2) | St. Jude Medical Standard 101 (934) | ||
| Edwards Lifesciences Perimount + 6900 TFX (2) | St. Jude Medical Masters 501/505 (303) | |||
| Epic Supra ESP E100 (5) | St. Jude Medical HP-Serie 105 (4) | |||
| Hancock II T505 (39) | Medtronic Hall A/M 7700 (58) | |||
| Hancock II T510 (672) | Duran 601H/608H (1) | |||
| Perimount Magna Mitral Ease 7300 TFX (18) | Carpentier-Edwards Classic 4400/4500 (3) | |||
| Sorin Pericarbon More (2) | Other bileaflet valves (4) | |||
| St. Jude Medical Epic ELS (6) | ||||
| Total Cohort | Biological | Mechanical | p Value | |
|---|---|---|---|---|
| Cardiac death * | 179/454 (39.4%) | 108/246 (43.9%) | 71/208 (34.1%) | 0.034 |
| Myocardial infarction | 36/651 (5.5%) | 17/319 (5.3%) | 19/332 (5.7%) | 0.826 |
| Reoperation | 10/651 (1.5%) | 5/319 (1.6%) | 5/332 (1.5%) | 0.949 |
| Other valve replacement | 13/651 (2%) | 4/319 (1.3%) | 9/332 (2.7%) | 0.184 |
| Bypass surgery | 4/651 (0.6%) | 2/319 (0.6%) | 2/332 (0.6%) | 0.968 |
| Pacemaker or Defibrillator | 134/651 (20.6%) | 67/319 (21%) | 67/332 (20.2%) | 0.970 |
| PTCA 1 or stents | 19/651 (2.9%) | 9/319 (2.8%) | 10/332 (3.0%) | 0.885 |
| Aortic operation | 1/651 (0.2%) | 1/319 (0.3%) | 0 | 0.49 ** |
| Ablation | 19/651 (2.9%) | 7/319 (2.2%) | 12/332 (3.6%) | 0.282 |
| Other cardiac intervention | 15/651 (2.3%) | 9/319 (2.8%) | 6/332 (1.8%) | 0.743 |
| Kidney disease | 167/651 (25.7%) | 100/319 (31.3%) | 67/332 (20.2%) | 0.001 |
| Embolic or thrombotic incidence | 48/651 (7.4%) | 29/319 (9.1%) | 19/332 (5.7%) | 0.100 |
| Postoperative bleeding | 53/651 (8.1%) | 15/319 (4.7%) | 38/332 (11.4%) | 0.002 |
| Stroke | 93/650 (14.3%) | 38/318 (11.9%) | 55/332 (16.6%) | 0.090 |
| AV block postop | 28/651 (4.3%) | 13/319 (4.1%) | 15/332 (4.5%) | 0.781 |
| Atrial fibrillation | 384/651 (59.0%) | 214/319 (67.1%) | 170/332 (51.2%) | <0.001 |
| NYHA | ||||
| NYHAI | 298/651 (45.8%) | 110/319 (34.5%) | 188/332 (56.6%) | <0.001 |
| NYHAII | 193/651 (29.6%) | 117/319 (36.7%) | 76/332 (22.9%) | <0.001 |
| NYHAIII | 144/651 (22.1%) | 83/319 (26%) | 61/332 (18.4%) | 0.019 |
| NYHAIV | 16/651 (2.5%) | 9/319 (2.8%) | 7/332 (2.1%) | 0.557 |
| Antithrombotic therapy | ||||
| Vitamin K antagonist | 484/645 (75.0%) | 160/319 (50.2%) | 324/326 (99.4%) | <0.001 |
| NOAC 2 | 64/651 (9.8%) | 63/319 (19.7%) | 1/332 (0.3%) | <0.001 |
| LMWH 3 | 14/651 (2.2%) | 8/319 (2.5%) | 6/332 (1.8%) | 0.538 |
| Platelet aggregation inhibitors | 74/651 (11.4%) | 72/319 (22.6%) | 2/332 (0.6%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veres, G.; Benke, K.; Stengl, R.; Weber, P.; Marina, E.; Szabó, G.; Karck, M. Long-Term Outcomes Stratified by Age in Patients with a Mechanical versus Biological Mitral Valve Replacement. J. Cardiovasc. Dev. Dis. 2022, 9, 339. https://doi.org/10.3390/jcdd9100339
Veres G, Benke K, Stengl R, Weber P, Marina E, Szabó G, Karck M. Long-Term Outcomes Stratified by Age in Patients with a Mechanical versus Biological Mitral Valve Replacement. Journal of Cardiovascular Development and Disease. 2022; 9(10):339. https://doi.org/10.3390/jcdd9100339
Chicago/Turabian StyleVeres, Gábor, Kálmán Benke, Roland Stengl, Petra Weber, Ereva Marina, Gábor Szabó, and Matthias Karck. 2022. "Long-Term Outcomes Stratified by Age in Patients with a Mechanical versus Biological Mitral Valve Replacement" Journal of Cardiovascular Development and Disease 9, no. 10: 339. https://doi.org/10.3390/jcdd9100339