The Effect of Habitat on Insect Movements: Experimental Evidence from Wild-Caught Butterflies
Abstract
:Simple Summary
Abstract
1. Introduction
- Does the level of spatial and temporal connectivity of L. camilla habitats vary across Wallonia? We expected (E1) that in this region there were areas with diverse habitat-change trajectories that could be pinpointed in space and time.
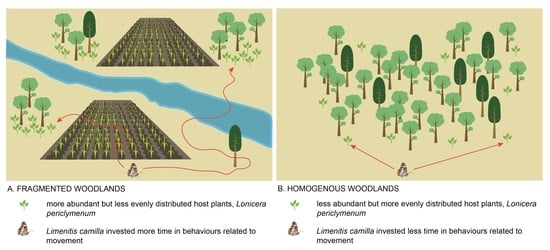
- Does host plant abundance and spatial distribution differ between homogenous vs. fragmented habitats? We expected (E2) that host plants were more abundant and more evenly distributed in homogenous compared to fragmented habitats.
- Do butterflies modify their behaviour due to the presence of the host plant in the experimental setup? We expected (E3) that butterflies spent relatively more time in the tunnel with the host plant than in the control tunnels, while accounting for abiotic factors that could have affected their movements in the tunnels.
- Do butterflies originating from fragmented vs. homogenous habitats differ in the time they allocate to different movement behaviours? We expected (E4) that individuals from fragmented habitats were more dispersive and spent less time navigating the tunnels in search of the host plant.
2. Material and Methods
2.1. The Model Species Limenitis camilla
2.2. Butterfly Occurrence Dataset
2.3. Functional Habitat Connectivity and Selection of Sampling Areas
Land Use Analysis
2.4. Sampling Populations of L. camilla and Its Host Plant L. periclymenum
2.5. Behavioural Experiments and Data Analyses
- “Departing”: flight activities associated with either butterflies repeatedly flying into the covering net of the tunnels or with flight towards the sun, a known tendency in butterflies. We interpreted these behaviours as the initiation of a dispersal movement from one patch of potential habitat (in our case, a patch inside the flight structure) to another [54,55]. We considered increased time spent in departing flight in butterflies as an indication of an increased investment in dispersal behaviour.
- “Navigation”: all behaviours involving interactions with the habitat inside the flight tunnels: flying freely in petal-like patterns, over/around the host plant, landing on the host plant, walking on the tunnel structure or on the ground (also defined “routine movements”, see [56]).
- “Resting”: sitting still on the net, on the tunnel structure or on the ground.
3. Results
3.1. Selection of Habitats with Low and High Levels of Functional Connectivity within Areas of Frequent L. camilla Observations (Expectation 1, E1)
3.2. Spatial Distribution and Abundance of the Host Plant (Expectation 2, E2)
3.3. Behavioural Activity of Populations from Fragmented vs. Homogenous Woodlands (Expectations 3 and 4, E3 and E4)
4. Discussion
4.1. Functional Habitat Connectivity and Host Plant Spatial Distribution (E1 and E2)
4.2. Behavioural Strategies of Populations from Fragmented vs. Homogenous Woodlands (E3 and E4)
4.3. Limitations and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- IPBES. Global assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Brondízio, E.S., Settele, J., Díaz, S., Ngo, H.T., Eds.; IPBES Secretariat: Bonn, Germany, 2019; 1144p, ISBN 978-3-947851-20-1. [Google Scholar]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive Human-Driven Decline of Life on Earth Points to the Need for Transformative Change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Ferrari, M.C.O.; Harris, D.J. Evolution and Behavioural Responses to Human-Induced Rapid Environmental Change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The Biomass Distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Warren, M.S.; Maes, D.; van Swaay, C.A.M.; Goffart, P.; Van Dyck, H.; Bourn, N.A.D.; Wynhoff, I.; Hoare, D.; Ellis, S. The Decline of Butterflies in Europe: Problems, Significance, and Possible Solutions. Proc. Natl. Acad. Sci. USA 2021, 118, e2002551117. [Google Scholar] [CrossRef] [PubMed]
- Van Swaay, C.; Maes, D.; Collins, S.; Munguira, M.L.; Šašić, M.; Settele, J.; Verovnik, R.; Warren, M.; Wiemers, M.; Wynhoff, I.; et al. Applying IUCN Criteria to Invertebrates: How Red Is the Red List of European Butterflies? Biol. Conserv. 2011, 144, 470–478. [Google Scholar] [CrossRef]
- Van Dyck, H.; Van Strien, A.J.; Maes, D.; Van Swaay, C.A.M. Declines in Common, Widespread Butterflies in a Landscape under Intense Human Use. Conserv. Biol. 2009, 23, 957–965. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Dennis, R.L.H. A Resource-Based Habitat View for Conservation: Butterflies in the British Landscape; Wiley-Blackwell: Chichester, UK, 2010; ISBN 978-1-4051-9945-2. [Google Scholar]
- Kotiaho, J.S.; Kaitala, V.; Komonen, A.; Päivinen, J. Predicting the Risk of Extinction from Shared Ecological Characteristics. Proc. Natl. Acad. Sci. USA 2005, 102, 1963–1967. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Hammerschlag, N.; Cooke, S.J.; Costa, D.P.; Irschick, D.J. Evolutionary Theory as a Tool for Predicting Extinction Risk. Trends Ecol. Evol. 2015, 30, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Mangels, J.; Fiedler, K.; Schneider, F.D.; Blüthgen, N. Diversity and Trait Composition of Moths Respond to Land-Use Intensification in Grasslands: Generalists Replace Specialists. Biodivers. Conserv. 2017, 26, 3385–3405. [Google Scholar] [CrossRef]
- Habel, J.C.; Trusch, R.; Schmitt, T.; Ochse, M.; Ulrich, W. Long-Term Large-Scale Decline in Relative Abundances of Butterfly and Burnet Moth Species across South-Western Germany. Sci. Rep. 2019, 9, 14921. [Google Scholar] [CrossRef]
- Wong, B.B.M.; Candolin, U. Behavioral Responses to Changing Environments. Behav. Ecol. 2014, 26, 665–673. [Google Scholar] [CrossRef]
- Candolin, U.; Wong, B.B. Behavioural Responses to a Changing World: Mechanisms and Consequences; Oxford University Press: Oxford, UK, 2012; ISBN 9780199602575. [Google Scholar]
- Bost, C.A.; Cotté, C.; Terray, P.; Barbraud, C.; Bon, C.; Delord, K.; Gimenez, O.; Handrich, Y.; Naito, Y.; Guinet, C.; et al. Large-Scale Climatic Anomalies Affect Marine Predator Foraging Behaviour and Demography. Nat. Commun. 2015, 6, 8220. [Google Scholar] [CrossRef]
- Prop, J.; Aars, J.; BÃ¥rdsen, B.-J.; Hanssen, S.A.; Bech, C.; Bourgeon, S.; de Fouw, J.; Gabrielsen, G.W.; Lang, J.; Noreen, E.; et al. Climate Change and the Increasing Impact of Polar Bears on Bird Populations. Front. Ecol. Evol. 2015, 3, 33. [Google Scholar] [CrossRef]
- Teitelbaum, C.S.; Converse, S.J.; Fagan, W.F.; Böhning-Gaese, K.; O’Hara, R.B.; Lacy, A.E.; Mueller, T. Experience Drives Innovation of New Migration Patterns of Whooping Cranes in Response to Global Change. Nat. Commun. 2016, 7, 12793. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.A.; Baskett, M.L.; Munch, S.B.; Hein, A.M. Fast Behavioral Feedbacks Make Ecosystems Sensitive to Pace and Not Just Magnitude of Anthropogenic Environmental Change. Proc. Natl. Acad. Sci. USA 2020, 117, 25580–25589. [Google Scholar] [CrossRef]
- Botero, C.A.; Weissing, F.J.; Wright, J.; Rubenstein, D.R. Evolutionary Tipping Points in the Capacity to Adapt to Environmental Change. Proc. Natl. Acad. Sci. USA 2014, 112, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Trimmer, P.C.; Ehlman, S.M. A Conceptual Framework for Understanding Behavioral Responses to HIREC. Curr. Opin. Behav. Sci. 2016, 12, 109–114. [Google Scholar] [CrossRef]
- Dukas, R. Cognitive Innovations and the Evolutionary Biology of Expertise. Philos. Trans. R. Soc. B 2017, 372, 20160427. [Google Scholar] [CrossRef] [PubMed]
- Snell-Rood, E.C.; Kobiela, M.E.; Sikkink, K.L.; Shephard, A.M. Mechanisms of Plastic Rescue in Novel Environments. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 331–354. [Google Scholar] [CrossRef]
- Ducatez, S.; Sol, D.; Sayol, F.; Lefebvre, L. Behavioural Plasticity Is Associated with Reduced Extinction Risk in Birds. Nat. Ecol. Evol. 2020, 4, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A Movement Ecology Paradigm for Unifying Organismal Movement Research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef]
- Bestion, E.; Cote, J.; Jacob, S.; Winandy, L.; Legrand, D. Habitat Fragmentation Experiments on Arthropods: What to Do Next? Curr. Opin. Insect Sci. 2019, 35, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Siffczyk, C.; Brotons, L.; Kangas, K.; Orell, M. Home Range Size of Willow Tits: A Response to Winter Habitat Loss. Oecologia 2003, 136, 635–642. [Google Scholar] [CrossRef]
- Evens, R.; Beenaerts, N.; Neyens, T.; Witters, N.; Smeets, K.; Artois, T. Proximity of Breeding and Foraging Areas Affects Foraging Effort of a Crepuscular, Insectivorous Bird. Sci. Rep. 2018, 8, 3008. [Google Scholar] [CrossRef]
- Northrup, J.M.; Anderson, C.R.; Wittemyer, G. Quantifying Spatial Habitat Loss from Hydrocarbon Development through Assessing Habitat Selection Patterns of Mule Deer. Glob. Chang. Biol. 2015, 21, 3961–3970. [Google Scholar] [CrossRef]
- Marchand, P.; Garel, M.; Bourgoin, G.; Duparc, A.; Dubray, D.; Maillard, D.; Loison, A. Combining Familiarity and Landscape Features Helps Break down the Barriers between Movements and Home Ranges in a Non-Territorial Large Herbivore. J. Anim. Ecol. 2017, 86, 371–383. [Google Scholar] [CrossRef]
- Goverde, M.; Schweizer, K.; Baur, B.; Erhardt, A. Small-Scale Habitat Fragmentation Effects on Pollinator Behaviour: Experimental Evidence from the Bumblebee Bombus Veteranus on Calcareous Grasslands. Biol. Conserv. 2002, 104, 293–299. [Google Scholar] [CrossRef]
- Dempster, J. Fragmentation, isolation and mobility of insect populations. In The Conservation of Insects and Their Habitats; Collins, N.M., Thomas, J.A., Eds.; Academic Press: London, UK, 1991; pp. 143–153. ISBN 978-0-12-181370-3. [Google Scholar]
- Hill, J.K.; Thomas, C.D.; Lewis, O.T. Flight Morphology in Fragmented Populations of a Rare British Butterfly, Hesperia Comma. Biol. Conserv. 1999, 87, 277–283. [Google Scholar] [CrossRef]
- Börschig, C.; Klein, A.-M.; von Wehrden, H.; Krauss, J. Traits of Butterfly Communities Change from Specialist to Generalist Characteristics with Increasing Land-Use Intensity. Basic Appl. Ecol. 2013, 14, 547–554. [Google Scholar] [CrossRef]
- Schtickzelle, N.; Joiris, A.; Van Dyck, H.; Baguette, M. Quantitative analysis of changes in movement behaviour within and outside habitat in a specialist butterfly. BMC Evol. Biol. 2007, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.; Van Dyck, H. Reproductive Plasticity, Oviposition Site Selection, and Maternal Effects in Fragmented Landscapes. Behav. Ecol. Sociobiol. 2009, 64, 1–11. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A Meta-Analysis of Preference-Performance Relationships in Phytophagous Insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Denno, R.F.; Larsson, S.; Olmstead, K.L. Role of Enemy-Free Space and Plant Quality in Host-Plant Selection by Willow Beetles. Ecology 1990, 71, 124–137. [Google Scholar] [CrossRef]
- Anderson, P.; Löfqvist, J. Asymmetric Oviposition Behaviour and the Influence of Larval Competition in the Two Pyralid Moths Ephestia Kuehniella and Plodia Interpunctella. Oikos 1996, 76, 47–56. [Google Scholar] [CrossRef]
- Villemey, A.; Peterman, W.E.; Richard, M.; Ouin, A.; van Halder, I.; Stevens, V.M.; Baguette, M.; Roche, P.; Archaux, F. Butterfly Dispersal in Farmland: A Replicated Landscape Genetics Study on the Meadow Brown Butterfly (Maniola jurtina). Landsc. Ecol. 2016, 31, 1629–1641. [Google Scholar] [CrossRef]
- Braem, S.; Turlure, C.; Nieberding, C.; Van Dyck, H. Oviposition Site Selection and Learning in a Butterfly under Niche Expansion: An Experimental Test. Anim. Behav. 2021, 180, 101–110. [Google Scholar] [CrossRef]
- Papaj, D.R. Ovarian Dynamics and Host Use. Annu. Rev. Entomol. 2000, 45, 423–448. [Google Scholar] [CrossRef]
- Catalogue of the Lepidoptera of Belgium. Available online: https://projects.biodiversity.be/lepidoptera (accessed on 10 August 2023).
- Bos, F.G.; Bosveld, M.A.; Groenendijk, D.G.; van Swaay, C.A.M.; Wynhoff, I. De Vlinderstichting. De Dagvlinders van Nederland, Verspreiding En Bescherming (Lepidoptera: Hesperioidea, Papilionoidea)—Nederlandse Fauna Deel 7; Naturalis, KNNV Uitgeverij, EIS: Leiden, Nederland, 2006; 348p. [Google Scholar] [CrossRef]
- Grashof-Bokdam, C.J.; Jansen, J.; Smulders, M.J.M. Dispersal Patterns of Lonicera Periclymenum Determined by Genetic Analysis. Molec. Ecol. 1998, 7, 165–174. [Google Scholar] [CrossRef]
- Fox, B.W. Alternative Foraging Strategies of the White Admiral Butterfly (Ladoga camilla L.) and the Broad Bordered Bee Hawk moth (Hemaris fuciformis L.) on Honeysuckle (Lonicera periclymenum L.). Ph.D. Thesis, University of Southampton, Southampton, UK, 1996. [Google Scholar]
- Fox, B. The larva of the White Admiral butterfly, Limenitis camilla (Linnaeus, 1764)—A master builder. Entomol. Gaz. 2005, 56, 225. [Google Scholar]
- Neteler, M.; Bowman, M.H.; Landa, M.; Metz, M. GRASS GIS: A Multi-Purpose Open Source GIS. Environ. Model. Softw. 2012, 31, 124–130. [Google Scholar] [CrossRef]
- Niebuhr, B.B.; Martello, F.; Ribeiro, J.W.; Vancine, M.H.; Muylaert, R.L.; Campos, V.E.W.; Silveira dos Santos, J.; Tonetti, V.R.; Ribeiro, M.C. Landscape Metrics (LSMetrics): A Tool for Calculating Landscape Connectivity and Other Ecologically Scaled Landscape Metrics; v0.91; Zenodo, 2020. [Google Scholar] [CrossRef]
- Matlack, G.R. Vegetation Dynamics of the Forest Edge—Trends in Space and Successional Time. J. Ecol. 1994, 82, 113. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T.G.; Van Dyck, H. Habitats and Resources: The Need for a Resource-Based Definition to Conserve Butterflies. Biodiv. Conserv. 2006, 15, 1943–1966. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T. Butterflies on British and Irish Offshore Islands: Ecology and Biogeography; Gem Publishing Company: Wallingford, UK, 1996; ISBN 978-0-906802-06-9. [Google Scholar]
- Ims, R.A.; Yoccoz, N.G. Studying Transfer Processes in Metapopulations: Emigration, Migration, and Colonization. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 247–265. [Google Scholar] [CrossRef]
- DiLeo, M.F.; Nonaka, E.; Husby, A.; Saastamoinen, M. Effects of Environment and Genotype on Dispersal Differ across Departure, Transfer and Settlement in a Butterfly Metapopulation. Proc. R. Soc. B 2022, 289, 20220322. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, H.; Baguette, M. Dispersal Behaviour in Fragmented Landscapes: Routine or Special Movements? Basic Appl. Ecol. 2005, 6, 535–545. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Usinglme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding Factors in the Detection of Species Responses to Habitat Fragmentation. Biol. Rev. 2005, 81, 117. [Google Scholar] [CrossRef]
- Chetcuti, J.; Kunin, W.E.; Bullock, J.M. Habitat Fragmentation Increases Overall Richness, but Not of Habitat-Dependent Species. Front. Ecol. Evol. 2020, 8, 607619. [Google Scholar] [CrossRef]
- Nieberding, C.M.; Van Dyck, H.; Chittka, L. Adaptive Learning in Non-Social Insects: From Theory to Field Work, and Back. Curr. Opin. Insect Sci. 2018, 27, 75–81. [Google Scholar] [CrossRef]
- Snell-Rood, E.C.; Ehlman, S.M. Ecology and Evolution of Plasticity. In Phenotypic Plasticity & Evolution; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Vries, J.P.R.; Koma, Z.; WallisDeVries, M.F.; Kissling, W.D. Identifying Fine-scale Habitat Preferences of Threatened Butterflies Using Airborne Laser Scanning. Divers. Distrib. 2021, 27, 1251–1264. [Google Scholar] [CrossRef]
- Woodbridge, J.; Roberts, N.; Fyfe, R. Vegetation and Land-Use Change in Northern Europe During Late Antiquity: A Regional-Scale Pollen-Based Reconstruction. Late Antiq. Archaeol. 2015, 11, 105–118. [Google Scholar] [CrossRef]
- Fuchs, R.; Herold, M.; Verburg, P.H.; Clevers, J.G.P.W.; Eberle, J. Gross Changes in Reconstructions of Historic Land Cover/Use for Europe between 1900 and 2010. Glob. Chang. Biol. 2014, 21, 299–313. [Google Scholar] [CrossRef]
- Fyfe, R.M.; Woodbridge, J.; Roberts, N. From Forest to Farmland: Pollen-inferred Land Cover Change across Europe Using the Pseudobiomization Approach. Glob. Chang. Biol. 2014, 21, 1197–1212. [Google Scholar] [CrossRef]
- Ustaoglu, E.; Collier, M. Farmland Abandonment in Europe: An Overview of Drivers, Consequences, and Assessment of the Sustainability Implications. Environ. Rev. 2018, 26, 396–416. [Google Scholar] [CrossRef]
- Singer, M.C. Adaptive and Maladaptive Consequences of Matching Habitat Choice: Lessons from a Rapidly-Evolving Butterfly Metapopulation. Evol. Ecol. 2015, 29, 905–925. [Google Scholar] [CrossRef]
- Kareiva, P. Experimental and Mathematical Analyses of Herbivore Movement: Quantifying the Influence of Plant Spacing and Quality on Foraging Discrimination. Ecol. Monogr. 1982, 52, 261–282. [Google Scholar] [CrossRef]
- Gibbs, M.; Breuker, C.J.; Hesketh, H.; Hails, R.S.; Van Dyck, H. Maternal Effects, Flight versus Fecundity Trade-Offs, and Offspring Immune Defence in the Speckled Wood Butterfly, Pararge Aegeria. BMC Evol. Biol. 2010, 10, 345. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Van Der Sterren, D.; Vastenhout, N.; Zwaan, B.J.; Brakefield, P.M. Predictive Adaptive Responses: Condition-Dependent Impact of Adult Nutrition and Flight in the Tropical Butterfly Bicyclus anynana. Am. Nat. 2010, 176, 686–698. [Google Scholar] [CrossRef]
- Bonte, D.; Van Dyck, H.; Bullock, J.M.; Coulon, A.; Del Mar Delgado, M.; Gibbs, M.; Lehouck, V.; Matthysen, E.; Mustin, K.; Saastamoinen, M.; et al. Costs of Dispersal. Biol. Rev. 2011, 87, 290–312. [Google Scholar] [CrossRef]
- Söderström, B.; Hedblom, M. Comparing Movement of Four Butterfly Species in Experimental Grassland Strips. J. Insect Conserv. 2006, 11, 333–342. [Google Scholar] [CrossRef]
- Srygley, R.B.; Kingsolver, J.G. Effects of Weight Loading on Flight Performance and Survival of Palatable Neotropical Anartia Fatima Butterflies. Biol. J. Linn. Soc. 2000, 70, 707–725. [Google Scholar] [CrossRef]
- Kissling, W.D.; Pattemore, D.E.; Hagen, M. Challenges and Prospects in the Telemetry of Insects. Biol. Rev. 2013, 89, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Kaláb, O.; Musiolek, D.; Rusnok, P.; Hurtik, P.; Tomis, M.; Kočárek, P. Estimating the Effect of Tracking Tag Weight on Insect Movement Using Video Analysis: A Case Study with a Flightless Orthopteran. PLoS ONE 2021, 16, e0255117. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Riabinina, O.; Potter, C.J. Olfactory Behaviors Assayed by Computer Tracking of Drosophila in a Four-Quadrant Olfactometer. J. Vis. Exp. 2016, 114, e54346. [Google Scholar] [CrossRef]







| Model Term | Estimate | Std. Error | z Value | Pr (>|z|) |
|---|---|---|---|---|
| Intercept | 3.62 | 0.21 | 17.46 | <0.001 |
| Tunnel TT | 2.05 | 0.36 | 5.94 | <0.001 |
| Departing (D) | −2.58 | 0.33 | −8.32 | <0.001 |
| Navigation (N) | −2.61 | 0.41 | −5.85 | <0.001 |
| TT:D | −0.12 | 0.55 | −0.43 | 0.831 |
| TT:N | 0.74 | 0.67 | 0.71 | 0.262 |
| Model Term | Estimate | Std. Error | z Value | Pr (>|z|) |
|---|---|---|---|---|
| Intercept | 6.76 | 0.22 | 31.26 | <0.001 |
| Origin FW | −0.37 | 0.36 | −1.02 | 0.311 |
| Departing (D) | −3.00 | 0.32 | −9.47 | <0.001 |
| Navigation (N) | −2.61 | 0.40 | −6.57 | <0.001 |
| Origin FW:Departing | 1.16 | 0.58 | 1.98 | 0.043 |
| Origin FW:Navigation | 0.08 | 0.58 | 0.14 | 0.892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcantonio, M.; Voda, R.; Da Re, D.; Igot, Q.; Dennis, R.L.H.; Vielfaure, A.; Vanwambeke, S.O.; Nieberding, C.M. The Effect of Habitat on Insect Movements: Experimental Evidence from Wild-Caught Butterflies. Insects 2023, 14, 737. https://doi.org/10.3390/insects14090737
Marcantonio M, Voda R, Da Re D, Igot Q, Dennis RLH, Vielfaure A, Vanwambeke SO, Nieberding CM. The Effect of Habitat on Insect Movements: Experimental Evidence from Wild-Caught Butterflies. Insects. 2023; 14(9):737. https://doi.org/10.3390/insects14090737
Chicago/Turabian StyleMarcantonio, Matteo, Raluca Voda, Daniele Da Re, Quentin Igot, Roger L. H. Dennis, Aurélien Vielfaure, Sophie O. Vanwambeke, and Caroline M. Nieberding. 2023. "The Effect of Habitat on Insect Movements: Experimental Evidence from Wild-Caught Butterflies" Insects 14, no. 9: 737. https://doi.org/10.3390/insects14090737