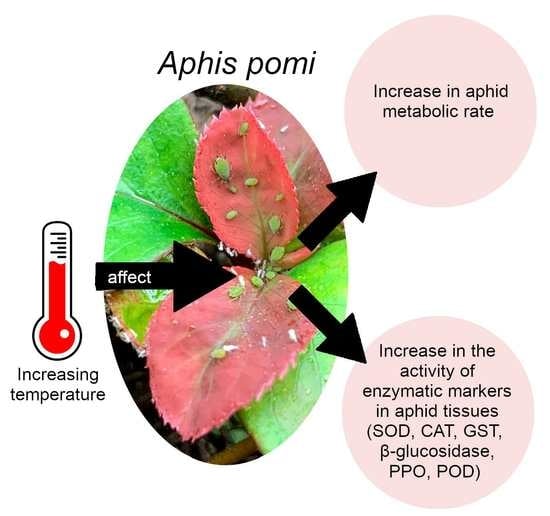
Enzymatic Defense Response of Apple Aphid Aphis pomi to Increased Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aphids
2.2. Host Plants
2.3. Effect of Temperature on Enzymatic Activity in Aphid Tissues
2.3.1. Homogenization of Aphids
2.3.2. Superoxide Dismutase (SOD) Activities
2.3.3. Catalase Activity (CAT)
2.3.4. β-Glucosidase Activity
2.3.5. Glutathione S-Transferase (GST) Activity
2.3.6. Polyphenol Oxidase (PPO) Activity
2.3.7. Peroxidase (POD) Activity
2.3.8. Protein Content in Aphid Tissue Homogenates
2.4. Effect of Temperature on Metabolic Activity of Aphids Using Isothermal Calorimetry
2.5. Statistical Analyses
3. Results
3.1. Effects of Temperature on Aphid Enzyme Activity
3.1.1. Superoxide Dismutase (SOD) and Catalase (CAT) Activity
3.1.2. β-Glucosidase and S-Glutathione Transferase (GST)
3.1.3. Polyphenol Oxidase (PPO) and Peroxidase (POD)
3.2. Determination of the Metabolic Activity of Aphids
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danks, H.V. The elements of seasonal adaptations in insects. Can. Entomol. 2007, 139, 1–44. [Google Scholar] [CrossRef]
- Hullé, M.; Coeur d’Acier, A.; Bankhead-Dronnet, S.; Harrington, R. Aphids in the face of global changes. Comptes Rendus Biol. 2010, 333, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Kiritani, K. A simple method to estimate the potential increase in the number of generations under global warming in temperate zones. Appl. Entomol. Zool. 1998, 33, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Durak, R.; Węgrzyn, E.; Leniowski, K. Do all aphids benefit from climate warming? An effect of temperature increase on a native species of temperate climatic zone Cinara juniperi. Ethol. Ecol. Evol. 2016, 28, 188–201. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Liu, H.; Qiao, G.; Huang, X. Investigating the impact of climate warming on phenology of aphid pests in China using long-term historical data. Insects 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durak, R.; Borowiak-Sobkowiak, B. Influence of temperature on the biological parameters of the anholocyclic species Cinara tujafilina (Hemiptera: Aphidoidea). Cent. Eur. J. Biol. 2013. [Google Scholar] [CrossRef]
- Kültz, D. DNA damage signals facilitate osmotic stress adaptation. Am. J. Physiol. Ren. Physiol. 2005, 289, F504–F505. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem. Mol. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef]
- Lalouette, L.; Williams, C.M.; Hervant, F.; Sinclair, B.J.; Renault, D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 229–234. [Google Scholar] [CrossRef]
- Matsumura, T.; Matsumoto, H.; Hayakawa, Y. Heat stress hardening of oriental armyworms is induced by a transient elevation of reactive oxygen species during sublethal stress. Arch. Insect Biochem. Physiol. 2017, 96, 1–10. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.; Li, N.; Zhang, F.; Liu, T.X. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant defense systems of two lipidopteran insect cell lines. Free Radic. Biol. Med. 2001. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D.; Turanli, F.; Sehnal, F. Stage-specific distribution of oxidative radicals and antioxidant enzymes in the midgut of Leptinotarsa decemlineata. J. Insect Physiol. 2007, 53, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, I.; Goławska, S. Effect of host plant on levels of reactive oxygen species andantioxidants in the cereal aphids Sitobion avenae and Rhopalosiphum padi. Biochem. Syst. Ecol. 2013, 51, 232–239. [Google Scholar] [CrossRef]
- Després, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Vanhaelen, N.; Haubruge, E. Glutathione S-transferases in the adaptation to plant secondary metabolites in the Myzus persicae aphid. Arch. Insect Biochem. Physiol. 2005, 58, 166–174. [Google Scholar] [CrossRef]
- Chrzanowski, G.; Leszczyński, B.; Czerniewicz, P.; Sytykiewicz, H.; Matok, H.; Krzyzanowski, R.; Sempruch, C. Effect of phenolic acids from black currant, sour cherry and walnut on grain aphid (Sitobion avenae F.) development. Crop Prot. 2012, 35, 71–77. [Google Scholar] [CrossRef]
- Łukasik, I.; Goławska, S.; Sytykiewicz, H.; Leszczyński, B. Antioxidant defence based on glutathione in grain aphid (Sitobion avenae (f.) and the bird cherry-oat aphid Rhopalosiphum padi: Responses to the host plant alteration. Allelopath. J. 2015, 35, 273–284. [Google Scholar]
- Krishnan, N.; Sehnal, F. Compartmentalization of oxidative stress and antioxidant defense in the larval gut of Spodoptera littoralis. Arch. Insect Biochem. Physiol. 2006, 63, 1–10. [Google Scholar] [CrossRef]
- Gupta, R.; Tara, J.S. Life history of Aphis pomi De Geer (green apple aphid) on apple plantations in Jammu Province, J&K, India. Munis Entomol. Zool. 2015, 10, 2–6. [Google Scholar]
- Kumari, M.; Gautam, D.C. Studies on the morphs, life history and behaviour of green apple aphid, Aphis pomi DE GEER on apple host in India. Polish J. Entomol. 2007, 76, 119–141. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, C. α-d-Glucosidase in the serum of the american cockroach. Periplaneta americana. Insect Biochem. 1979. [Google Scholar] [CrossRef]
- Leszczynski, B.; Dixon, A.F.G. Resistance of cereals to aphids: The interaction between hydroxamic acids and glutathione S-transferases in the grain aphid Sitobion avenae (F.) (Hom., Aphididae). J. Appl. Entomol. 1992, 113, 61–67. [Google Scholar] [CrossRef]
- Miles, P.W. Studies on the salivary physiology of plant bugs: Oxidase activity in the salivary apparatus and saliva. J. Insect Physiol. 1964, 10, 121–129. [Google Scholar] [CrossRef]
- Laurema, S.; Varis, A.L.; Miettinen, H. Studies on enzymes in the salivary glands of Lygus rugulipennis (Hemiptera, miridae). Insect Biochem. 1985, 15, 211–224. [Google Scholar] [CrossRef]
- Fehrman, H.; Dimond, A.E. Peroxidase activity and phytophthora resistance in different organs of the potato plant. Phytopathology 1969, 57, 69–72. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with Folin Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bi, J.L.; Felton, G.W. Foliar oxidative stress and insect herbivory: Primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J. Chem. Ecol. 1995, 21, 1511–1530. [Google Scholar] [CrossRef]
- Durak, R.; Bednarski, W.; Formela-Luboińska, M.; Woźniak, A.; Borowiak-Sobkowiak, B.; Durak, T.; Dembczyński, R.; Morkunas, I. Defense responses of Thuja orientalis to infestation of anholocyclic species aphid Cinara tujafilina. J. Plant Physiol. 2018, 232, 160–170. [Google Scholar] [CrossRef]
- Lukasik, I. Changes in activity of superoxide dismutase and catalase within cereal aphids in response to plant o-dihydroxyphenols. J. Appl. Entomol. 2007, 131, 209–214. [Google Scholar] [CrossRef]
- Abdelsalam, S.A.; Awad, A.M.A.; Abdelrahman, M.A.A.; Nasser, M.A.K.; Abdelhamid, N.M.R. Antioxidant defense response of the green peach aphid, Myzus persicae against secondary metabolites of the host plants cumin, anise, and coriander. J. Agric. Sci. Technol. 2016, 18, 1583–1592. [Google Scholar]
- Barbehenn, R.V. Gut-based antioxidant enzymes in a polyphagous and a graminivorous grasshopper. J. Chem. Ecol. 2002, 28, 1329–1347. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Rashid, M.A.; Huang, Q.Y.; Wong, C.; Lei, C.L. Response of antioxidant enzymes in Mythimna separata (Lepidoptera: Noctuidae) exposed to thermal stress. Bull. Entomol. Res. 2017, 107, 382–390. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Liu, X.; Yu, W.B.; Tan, X.L.; Zhang, S.Z.; Tian, H.G.; Liu, T.X. The potential coordination of the heat-shock proteins and antioxidant enzyme genes of Aphidius gifuensis in response to thermal stress. Front. Physiol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jena, K.; Kumar Kar, P.; Kausar, Z.; Babu, C.S. Effects of temperature on modulation of oxidative stress and antioxidant defenses in testes of tropical tasar silkworm Antheraea mylitta. J. Therm. Biol. 2013, 38, 199–204. [Google Scholar] [CrossRef]
- Durak, R.; Molon, M.; Durak, T.; Chrzanowski, G. The enzymatic markers of the adaptation of Cinara tujafilina to changing the host plant. Ethol. Ecol. Evol. 2018, 30, 416–429. [Google Scholar] [CrossRef]
- Łukasik, I. Effect of host plant alternation on some adaptive enzymes of the bird cherry—Oat aphid, Rhopalosiphum padi (L.). J. Pest Sci. (2004) 2009, 82, 203–209. [Google Scholar] [CrossRef]
- Mehrabadi, M.; Bandani, A.R.; Kwon, O. Biochemical characterization of digestive α-d-glucosidase and β-d-glucosidase from labial glands and midgut of wheat bug Eurygaster maura (Hemiptera: Scutelleridae). Entomol. Res. 2011, 41, 81–87. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving potato stress tolerance and tuber yield under a climate change scenario—A current overview. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Hao, T.; Jin, H.; Zhang, H.; He, L.; Zhou, Q.; Huang, D.; Hui, D.; Yu, J. Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L). PLoS ONE 2016, 11, e0152429. [Google Scholar] [CrossRef] [PubMed]
- Dancewicz, K.; Paprocka, M.; Morkunas, I.; Gabryś, B. Struggle to survive: Aphid—plant relationships under low-light stress. A case of Acyrthosiphon pisum (Harris) and Pisum sativum L. Arthropod. Plant. Interact. 2018, 12, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Urbanska, A.; Freddy Tjallingii, W.; Dixon, A.F.G.; Leszczynski, B. Phenol oxidising enzymes in the grain aphid’s saliva. Entomol. Exp. Appl. 1998, 86, 197–203. [Google Scholar] [CrossRef]
- Robinson, J.M.; Karnovsky, M.J.; Stoward, P.J.; Lewis, P.R. Oxidases. In Histochemistry, Theoretical and Applied, 2nd ed.; Stoward, P., Pearse, A.G.E., Eds.; Churchill and Livingstone: Edinburgh, UK, 1991; Volume 3, pp. 95–115. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Phytochemistry: Advances in Research; Research Signpost: Trivandrum, India, 2006; Volume 661, ISBN 8130800349. [Google Scholar]
- Cai, Q.N.; Han, Y.; Cao, Y.Z.; Hu, Y.; Zhao, X.; Bi, J.L. Detoxification of gramine by the cereal aphid Sitobion avenae. J. Chem. Ecol. 2009, 35, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Durak, R.; Dampc, J.; Dampc, J. Role of temperature on the interaction between Japanese quince Chaenomeles japonica and herbivorous insect Aphis pomi (Hemiptera: Aphidoidea). Environ. Exp. Bot. 2020, 176, 104100. [Google Scholar] [CrossRef]
- Joyal, J.J.; Hansen, L.D.; Coons, D.R.; Booth, G.M.; Smith, B.N.; Mill, D.D. Calorespirometric determination of the effects of temperature, humidity, low O2 and high CO2 on the development of Musca domestica pupae. J. Therm. Anal. Calorim. 2005, 82, 703–709. [Google Scholar] [CrossRef]
- Borowiak-Sobkowiak, B. Effect of temperature on the biological parameters of Aphis craccivora (Hemiptera Aphididae) on Robinia pseudoacacia. Redia 2017, 100, 65–71. [Google Scholar] [CrossRef]
- Mehrparvar, M.; Hatami, B. Effect of temperature on some biological parameters of an Iranian population of the rose aphid, Macrosiphum rosae (Hemiptera: Aphididae). Eur. J. Entomol. 2007, 104, 631–634. [Google Scholar] [CrossRef]
- Wang, J.-J.; Tsai, J.H. Effect of Temperature on the biology of Aphis spiraecola (Homoptera: Aphididae). Ann. Entomol. Soc. Am. 2000, 93, 874–883. [Google Scholar] [CrossRef]
- Son, Y.; Johnson, M.W.; Backus, E.A.; Groves, R.L. Pattern of stylet penetration activity by Homalodisca vitripennis (Hemiptera: Cicadellidae) adults in relation to environmental temperature and light conditions. Environ. Entomol. 2012, 41, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.J.; Webster, J.A. Probing Behavior of Diuraphis noxia and Rhopalosiphum maidis (Homoptera: Aphididae) Affected by Barley Resistance to D. noxia and Plant Water Stress. Environ. Entomol. 2001, 30, 1041–1046. [Google Scholar] [CrossRef] [Green Version]
- Dáder, B.; Fereres, A.; Moreno, A.; Trȩbicki, P. Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Criddle, R.S.; Mitcham, E.J. Metabolic response of Platynota stultana pupae to controlled atmospheres and its relation to insect mortality response. J. Insect Physiol. 2000, 46, 1375–1385. [Google Scholar] [CrossRef]
- Zhou, S.; Criddle, R.S.; Mitcham, E.J. Metabolic response of Platynota stultana pupae during and after extended exposure to elevated CO2 and reduced O2 atmospheres. J. Insect Physiol. 2001, 47, 401–409. [Google Scholar] [CrossRef]
- Neven, L.G.; Lehrman, N.J.; Hansen, L.D. Effects of temperature and modified atmospheres on diapausing 5th instar codling moth metabolism. J. Therm. Biol. 2014. [Google Scholar] [CrossRef]
- Schmolz, E.; Dewitz, R.; Schricker, B.; Lamprecht, I. Energy metabolism of European (Apis mellifera carnica) and Egyptian (A. m. lamarckii) honeybees. J. Therm. Anal. Calorim. 2001, 65, 131–140. [Google Scholar] [CrossRef]
- Schmolz, E.; Geisenheyner, S.; Schricker, B.; Lamprecht, I. Heat dissipation of flying wax moths (Galleria mellonella) measured by means of direct calorimetry. J. Therm. Anal. Calorim. 1999. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Y.; Huang, Z.; Huang, J. The effect on the longevity of Leptocybe invasa Fisher & LaSalle (Hymenoptera: Eulophidae) studied by microcalorimetry and traditional methods. J. Therm. Anal. Calorim. 2014, 116, 461–467. [Google Scholar] [CrossRef]
- Hussain, M.; Lin, Y.; Wang, L. Effect of temperature on longevity of Diaphorina citri (Hemiptera: Liviidae) studied by microcalorimeter. J. Therm. Anal. Calorim. 2017, 127, 1245–1252. [Google Scholar] [CrossRef]
- Williams, R.S.; Lincoln, D.E.; Norby, R.J. Development of gypsy moth larvae feeding on red maple saplings at elevated CO2 and temperature. Oecologia 2003, 137, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kiritani, K. Predicting impacts of global warming on population dynamics and distribution of arthropods in Japan. Popul. Ecol. 2006, 48, 5–12. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Forister, M.L.; Shapiro, A.M. Climatic trends and advancing spring flight of butterflies in lowland California. Glob. Chang. Biol. 2003, 9, 1130–1135. [Google Scholar] [CrossRef]
- Harmon, J.P.; Moran, N.A.; Ives, A.R. Species Response to Environmental Change: Impacts of Food Web Interactions and Evolution. Science 2009, 323, 1347–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boher, F.; Trefault, N.; Piulachs, M.D.; Bellés, X.; Godoy-Herrera, R.; Bozinovic, F. Biogeographic origin and thermal acclimation interact to determine survival and hsp90 expression in Drosophila species submitted to thermal stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 162, 391–396. [Google Scholar] [CrossRef]
- Arnal, P.; Coeur d’acier, A.; Favret, C.; Godefroid, M.; Qiao, G.X.; Jousselin, E.; Sanchez Meseguer, A. The evolution of climate tolerance in conifer-feeding aphids in relation to their host’s climatic niche. Ecol. Evol. 2019, 9, 11657–11671. [Google Scholar] [CrossRef]
- Durak, R. The overwintering strategy of the anholocyclic aphid Cinara tujafilina. Physiol. Entomol. 2014, 39, 313–321. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic Defense Response of Apple Aphid Aphis pomi to Increased Temperature. Insects 2020, 11, 436. https://doi.org/10.3390/insects11070436
Dampc J, Kula-Maximenko M, Molon M, Durak R. Enzymatic Defense Response of Apple Aphid Aphis pomi to Increased Temperature. Insects. 2020; 11(7):436. https://doi.org/10.3390/insects11070436
Chicago/Turabian StyleDampc, Jan, Monika Kula-Maximenko, Mateusz Molon, and Roma Durak. 2020. "Enzymatic Defense Response of Apple Aphid Aphis pomi to Increased Temperature" Insects 11, no. 7: 436. https://doi.org/10.3390/insects11070436