Effects of Mycobacterium vaccae NCTC 11659 and Lipopolysaccharide Challenge on Polarization of Murine BV-2 Microglial Cells
Abstract
:1. Introduction
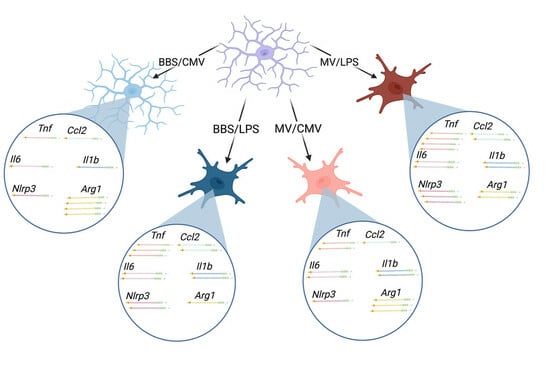
2. Results
2.1. Experiment 1: LPS Dose–Response
2.2. Experiment 2: Principal Coordinate Analysis of All Samples Used for the NanoString Platform
2.3. Effects of M. vaccae NCTC 11659 on mRNA Expression in BV-2 Microglial Cells Based on Analysis Using the NanoString Platform
2.4. Effects of LPS on mRNA Expression in Murine BV-2 Microglial Cells Based on Analysis Using the NanoString Platform
2.5. Differential Gene Expression between M. vaccae NCTC 11659/LPS as Compared to BBS/LPS Based on Analysis Using the NanoString Platform
2.6. Differential Gene Expression between M. vaccae NCTC 11659/LPS as Compared to M. vaccae NCTC 11659/CMV Based on Analysis Using the NanoString Platform
2.7. Validation of Effects of M. vaccae NCTC 11659 and LPS on Arg1 Gene Expression in BV-2 Microglial Cells Using Real-Time RT-PCR
2.8. Validation of Effects of M. vaccae NCTC 11659 and LPS on Genes Encoding Proinflammatory Cytokines and Chemokine Ligands (i.e., Il1b, Il6, Tnf, and Ccl2 Gene Expression) in BV-2 Microglial Cells Using Real-Time RT-PCR
2.9. Validation of Effects of M. vaccae NCTC 11659 and LPS on Nlrp3 mRNA Expression in BV-2 Microglial Cells Using Real-Time RT-PCR
3. Discussion
3.1. M. vaccae NCTC 11659 by Itself Polarized Murine BV-2 Microglial Cells toward a Proinflammatory Phenotype
3.2. LPS Strongly Polarizes Murine BV-2 Microglial Cells toward a Proinflammatory Phenotype
3.3. M. vaccae NCTC 11659 Enhanced the Polarizing Effects of LPS on Inflammatory mRNA Expression in Murine BV-2 Microglial Cells, Consistent with Neuroinflammatory and Microglial Priming
3.4. M. vaccae NCTC 11659 Attenuated LPS-Induced Reduction in Arg1 Gene Expression
3.5. Comparisons of the Effects of M. vaccae NCTC 11659 on the Immunophenotype of Microglia Following Administration In Vivo versus In Vitro
3.6. Limitations
3.7. Clinical Implications
3.8. Conclusions and Future Directions
4. Materials and Methods
4.1. Murine BV-2 Microglial Cells
4.2. M. vaccae NCTC 11659
4.3. LPS
4.4. Experimental Timeline
4.5. RNA Isolation
4.6. NanoString nCounter Gene Expression
4.7. Real-Time RT-PCR and Primers
4.8. Statistical Analysis
4.8.1. NanoString Analysis
4.8.2. Real-Time RT-PCR Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Koenen, K.C.; Ratanatharathorn, A.; Ng, L.; McLaughlin, K.A.; Bromet, E.J.; Stein, D.J.; Karam, E.G.; Meron Ruscio, A.; Benjet, C.; Scott, K.; et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 2017, 47, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med. 2014, 76, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W.; Lowry, C.A. (Eds.) Evolution, Biodiversity and a Reassessment of the Hygiene Hypothesis; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- Amoroso, M.; Langgartner, D.; Lowry, C.A.; Reber, S.O. Rapidly growing Mycobacterium species: The long and winding road from tuberculosis vaccines to potent stress-resilience agents. Int. J. Mol. Sci. 2021, 22, 12938. [Google Scholar] [CrossRef] [PubMed]
- Ellul, P.; Mariotti-Ferrandiz, E.; Leboyer, M.; Klatzmann, D. Regulatory T cells as supporters of psychoimmune resilience: Toward immunotherapy of major depressive disorder. Front. Neurol. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R.F.; Martinelli, R.; Adams, V.C.; Rook, G.A.W.; Brunet, L.R. Intragastric administration of Mycobacterium vaccae inhibits severe pulmonary allergic inflammation in a mouse model. Clin. Exp. Allergy 2005, 35, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Zuany-Amorim, C.; Manlius, C.; Trifilieff, A.; Brunet, L.R.; Rook, G.; Bowen, G.; Pay, G.; Walker, C. Long-term protective and antigen-specific effect of heat-killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J. Immunol. 2002, 169, 1492–1499. [Google Scholar] [CrossRef]
- Zuany-Amorim, C.; Sawicka, E.; Manlius, C.; Le Moine, A.; Brunet, L.R.; Kemeny, D.M.; Bowen, G.; Rook, G.; Walker, C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 2002, 8, 625–629. [Google Scholar] [CrossRef]
- Reber, S.O.; Siebler, P.H.; Donner, N.C.; Morton, J.T.; Smith, D.G.; Kopelman, J.M.; Lowe, K.R.; Wheeler, K.J.; Fox, J.H.; Hassell, J.E., Jr.; et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E3130–E3139. [Google Scholar] [CrossRef]
- Fonken, L.K.; Frank, M.G.; D’Angelo, H.M.; Heinze, J.D.; Watkins, L.R.; Lowry, C.A.; Maier, S.F. Mycobacterium vaccae immunization protects aged rats from surgery-elicited neuroinflammation and cognitive dysfunction. Neurobiol. Aging 2018, 71, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Fonken, L.K.; Dolzani, S.D.; Annis, J.L.; Siebler, P.H.; Schmidt, D.; Watkins, L.R.; Maier, S.F.; Lowry, C.A. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: Attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav. Immun. 2018, 73, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Fonken, L.K.; Watkins, L.R.; Maier, S.F.; Lowry, C.A. Could probiotics be used to mitigate neuroinflammation? ACS Chem. Neurosci. 2019, 10, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, K.; Darling, J.S.; Kakkar, R.; Wu, S.L.; Zentay, A.; Lowry, C.A.; Fonken, L.K. Mycobacterium vaccae immunization in rats ameliorates features of age-associated microglia activation in the amygdala and hippocampus. Sci. Rep. 2022, 12, 2165. [Google Scholar] [CrossRef] [PubMed]
- Narwaria, M.; Shah, V.; Patel, P.S.; Patel, S.; Aswani, V. Early experience of high-dose intravenous Mycobacterium w in critically ill patients of COVID-19. Indian J. Crit. Care Med. 2021, 25, 1066–1068. [Google Scholar] [CrossRef]
- Sehgal, I.S.; Guleria, R.; Singh, S.; Siddiqui, M.S.; Agarwal, R. A randomised trial of Mycobacterium w in critically ill patients with COVID-19: ARMY-1. ERJ Open Res. 2021, 7, 00059–2021. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H.; Hughes, T.K.; Pokkali, S.; Swanson, P.A.; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef]
- Holbrook, E.M.; Zambrano, C.A.; Wright, C.T.O.; Dubé, E.M.; Stewart, J.R.; Sanders, W.J.; Frank, M.G.; MacDonald, A.S.; Reber, S.O.; Lowry, C.A. Mycobacterium vaccae NCTC 11659, a soil-derived bacterium with stress resilience properties, modulates the proinflammatory effects of LPS in macrophages. Int. J. Mol. Sci. 2023, 24, 5176. [Google Scholar] [CrossRef]
- Loupy, K.M.; Cler, K.E.; Marquart, B.M.; Yifru, T.W.; D’Angelo, H.M.; Arnold, M.R.; Elsayed, A.I.; Gebert, M.J.; Fierer, N.; Fonken, L.K.; et al. Comparing the effects of two different strains of mycobacteria, Mycobacterium vaccae NCTC 11659 and M. vaccae ATCC 15483, on stress-resilient behaviors and lipid-immune signaling in rats. Brain Behav. Immun. 2021, 91, 212–229. [Google Scholar] [CrossRef]
- You, M.M.; Chen, Y.F.; Pan, Y.M.; Liu, Y.C.; Tu, J.; Wang, K.; Hu, F.L. Royal Jelly Attenuates LPS-Induced Inflammation in BV-2 Microglial Cells through Modulating NF-kappaB and p38/JNK Signaling Pathways. Mediat. Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.Y.; Oh, E.S.; Oh, S.; Han, I.O. IL-1beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappaB pathways. J. Neurosci. Res. 2006, 84, 1037–1046. [Google Scholar] [CrossRef]
- Tan, J.; Li, W.; Teng, Z.; Wang, G.; Li, Y.; Zhang, Y. Senkyunolide H inhibits activation of microglia and attenuates lipopolysaccharide-mediated neuroinflammation and oxidative stress in BV2 microglia cells via regulating ERK and NF-kappaB pathway. Kaohsiung J. Med. Sci. 2022, 38, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Taetzsch, T.; Levesque, S.; McGraw, C.; Brookins, S.; Luqa, R.; Bonini, M.G.; Mason, R.P.; Oh, U.; Block, M.L. Redox regulation of NF-kappaB p50 and M1 polarization in microglia. Glia 2015, 63, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Lowry, C.; Hollis, J.; de Vries, A.; Pan, B.; Brunet, L.; Hunt, J.; Paton, J.; van Kampen, E.; Knight, D.; Evans, A.; et al. Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior. Neuroscience 2007, 146, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Abdollah, S.; Qiu, Y.; Cai, J.; Xu, Y.Y.; Grinnell, B.W.; Richardson, M.A.; Topper, J.N.; Gimbrone, M.A., Jr.; Wrana, J.L.; et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 1997, 89, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Gaekwad, J.; Zhang, Y.; Zhang, W.; Reeves, J.; Wolfert, M.A.; Boons, G.-J. Differential induction of innate immune responses by synthetic lipid A derivatives. J. Biol. Chem. 2010, 285, 29375–29386. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Chiba, K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol. Ther. 2015, 154, 21–35. [Google Scholar] [CrossRef]
- Weber, M.D.; Frank, M.G.; Tracey, K.J.; Watkins, L.R.; Maier, S.F. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: A priming stimulus of microglia and the NLRP3 inflammasome. J. Neurosci. 2015, 35, 316–324. [Google Scholar] [CrossRef]
- Cunha, C.; Gomes, C.; Vaz, A.R.; Brites, D. Exploring new inflammatory biomarkers and pathways during LPS-induced M1 polarization. Mediat. Inflamm. 2016, 2016, 6986175. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-C.; Zheng, M.-H.; Du, Y.-L.; Wang, L.; Kuang, F.; Qin, H.-Y.; Zhang, B.-F.; Han, H. N9 microglial cells polarized by LPS and IL4 show differential responses to secondary environmental stimuli. Cell Immunol. 2012, 278, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Fenn, A.M.; Henry, C.J.; Huang, Y.; Dugan, A.; Godbout, J.P. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav. Immun. 2012, 26, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of general and discriminating markers of differential microglia phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhai, H.; Wang, Y.; Li, L.; Wu, J.; Wang, F.; Sun, S.; Yao, S.; Shang, Y. Aspirin-triggered lipoxin A(4) attenuates lipopolysaccharide-induced intracellular ROS in BV2 microglia cells by inhibiting the function of NADPH oxidase. Neurochem. Res. 2012, 37, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Vieyra, R.; Silva-Garcia, O.; Gomez-Garcia, A.; Gutierrez-Castellanos, S.; Alvarez-Aguilar, C.; Baizabal-Aguirre, V.M. Glycogen synthase kinase 3beta modulates the inflammatory response activated by bacteria, viruses, and parasites. Front. Immunol. 2021, 12, 675751. [Google Scholar] [CrossRef] [PubMed]
- Vizzardelli, C.; Pavelka, N.; Luchini, A.; Zanoni, I.; Bendickson, L.; Pelizzola, M.; Beretta, O.; Foti, M.; Granucci, F.; Nilsen-Hamilton, M.; et al. Effects of dexamethazone on LPS-induced activation and migration of mouse dendritic cells revealed by a genome-wide transcriptional analysis. Eur. J Immunol. 2006, 36, 1504–1515. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Cui, Z.; Yang, W.; Yue, L.; Ma, Y.; Shi, S.; Wang, C.; Qian, A. Mycobacterium vaccae induces a strong Th1 response that subsequently declines in C57BL/6 mice. J. Vet. Sci. 2016, 17, 505–513. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Arginase 1+ microglia reduce Aβ plaque deposition during IL-1β-dependent neuroinflammation. J. Neuroinflamm. 2015, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, L.M.; Boot, M.; Kuijl, C.; Picavet, D.I.; van Stempvoort, G.; van der Pol, S.M.A.; de Vries, H.E.; van der Wel, N.N.; van der Kuip, M.; van Furth, A.M.; et al. Mycobacteria employ two different mechanisms to cross the blood-brain barrier. Cell Microbiol. 2018, 20, e12858. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Tobin, D.M.; Tucker, E.W.; Venketaraman, V.; Ordonez, A.A.; Jayashankar, L.; Siddiqi, O.K.; Hammoud, D.A.; Prasadarao, N.V.; Sandor, M.; et al. Tuberculous meningitis: A roadmap for advancing basic and translational research. Nat. Immunol. 2018, 19, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Garibay, C.; Salinas-Lara, C.; Gómez-López, M.A.; Soto-Rojas, L.O.; Castillón-Benavides, N.K.; Castillón-Benavides, O.J.; Hernández-Campos, M.E.; Hernández-Pando, R.; Marquina-Castillo, B.; Flores-Barrada, M.A.; et al. Mycobacterium tuberculosis infection induces BCSFB disruption but no BBB disruption in vivo: Implications in the pathophysiology of tuberculous meningitis. Int. J. Mol. Sci. 2022, 23, 6436. [Google Scholar] [CrossRef] [PubMed]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Henn, A.; Lund, S.; Hedtjarn, M.; Schrattenholz, A.; Porzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Westfall, S.; Caracci, F.; Zhao, D.; Wu, Q.L.; Frolinger, T.; Simon, J.; Pasinetti, G.M. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav. Immun. 2021, 91, 350–368. [Google Scholar] [CrossRef]
- Lam, D.; Lively, S.; Schlichter, L.C. Responses of rat and mouse primary microglia to pro- and anti-inflammatory stimuli: Molecular profiles, K(+) channels and migration. J. Neuroinflamm. 2017, 14, 166. [Google Scholar] [CrossRef]
- Kessal, K.; Liang, H.; Rabut, G.; Daull, P.; Garrigue, J.-S.; Docquier, M.; Parsadaniantz, S.M.; Baudouin, C.; Brignole-Baudouin, F. Conjunctival inflammatory gene expression profiling in dry eye disease: Correlations with HLA-DRA and HLA-DRB1. Front. Immunol. 2018, 9, 2271. [Google Scholar] [CrossRef]
- Izzy, S.; Liu, Q.; Fang, Z.; Lule, S.; Wu, L.; Chung, J.Y.; Sarro-Schwartz, A.; Brown-Whalen, A.; Perner, C.; Hickman, S.E.; et al. Time-dependent changes in microglia transcriptional networks following traumatic brain injury. Front. Cell. Neurosci. 2019, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Ermann, J.; Matmusaev, M.; Haley, E.K.; Braun, C.; Jost, F.; Mayer-Wrangowski, S.; Hsiao, P.; Ting, N.; Li, L.; Terenzio, D.J.; et al. The potent and selective RIPK2 inhibitor BI 706039 improves intestinal inflammation in the TRUC mouse model of inflammatory bowel disease. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 321, G500–G512. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Taylor, L.; Feuerborn, A.; Valaris, S.; Hussain, M.T.; Rainger, G.E.; Greaves, D.R.; Iqbal, A.J. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB J. 2019, 33, 6154–6167. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, E.; Brown, F.N.; Shula, C.; Kahn, F.; Lee, S.H.; Berta, T.; Ladle, D.R.; Campbell, K.; Mangano, F.T.; Goto, J. The anti-inflammatory agent bindarit attenuates the impairment of neural development through suppression of microglial activation in a neonatal hydrocephalus mouse model. J. Neurosci. 2022, 42, 1820–1844. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.B.; Lopes, M.E.; Hohman, L.S.; Romano, A.; David, B.A.; Kratofil, R.; Kubes, P.; Workentine, M.L.; Campos, A.C.; Vieira, L.Q.; et al. Th1-Th2 cross-regulation controls early Leishmania infection in the skin by modulating the size of the permissive monocytic host cell reservoir. Cell Host Microbe 2020, 27, 752–768. [Google Scholar] [CrossRef] [PubMed]
- Sunuwar, L.; Frkatović, A.; Sharapov, S.; Wang, Q.; Neu, H.M.; Wu, X.; Haritunians, T.; Wan, F.; Michel, S.; Wu, S.; et al. Pleiotropic ZIP8 A391T implicates abnormal manganese homeostasis in complex human disease. JCI Insight 2020, 5, e140978. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Manangeeswaran, M.; Lewkowicz, A.P.; Engel, K.; Chowdhury, M.; Garige, M.; Eckhaus, M.A.; Sourbier, C.; Ireland, D.D.; Verthelyi, D. NK cells require immune checkpoint receptor LILRB4/gp49B to control neurotropic Zika virus infections in mice. JCI Insight 2022, 7, e151420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gillaspy, A.F.; Gipson, J.R.; Cassidy, B.R.; Nave, J.L.; Brewer, M.F.; Stoner, J.A.; Chen, J.; Drevets, D.A. Neuroinvasive Listeria monocytogenes infection triggers IFN-activation of microglia and upregulates microglial miR-155. Front. Immunol. 2018, 9, 2751. [Google Scholar] [CrossRef]
- Elchaninov, A.; Nikitina, M.; Vishnyakova, P.; Lokhonina, A.; Makarov, A.; Sukhikh, G.; Fatkhudinov, T. Macro- and microtranscriptomic evidence of the monocyte recruitment to regenerating liver after partial hepatectomy in mouse model. Biomed. Pharmacother. 2021, 138, 111516. [Google Scholar] [CrossRef]
- Yau, C.; Gan, E.S.; Kwek, S.S.; Tan, H.C.; Ong, E.Z.; Hamis, N.Z.; Rivino, L.; Chan, K.R.; Watanabe, S.; Vasudevan, S.G.; et al. Live vaccine infection burden elicits adaptive humoral and cellular immunity required to prevent Zika virus infection. eBioMedicine 2020, 61, 103028. [Google Scholar] [CrossRef]
- Niraula, A.; Witcher, K.G.; Sheridan, J.F.; Godbout, J.P. Interleukin-6 induced by social stress promotes a unique transcriptional signature in the monocytes that facilitate anxiety. Biol. Psychiatry 2019, 85, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Senra, L.; Mylonas, A.; Kavanagh, R.D.; Fallon, P.G.; Conrad, C.; Borowczyk-Michalowska, J.; Wrobel, L.J.; Kaya, G.; Yawalkar, N.; Boehncke, W.H.; et al. IL-17E (IL-25) enhances innate immune responses during skin inflammation. J. Investig. Dermatol. 2019, 139, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Broggi, A.; Tan, Y.; Granucci, F.; Zanoni, I. IFN-lambda suppresses intestinal inflammation by non-translational regulation of neutrophil function. Nat. Immunol. 2017, 18, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Witcher, K.G.; Bray, C.E.; Dziabis, J.E.; McKim, D.B.; Benner, B.N.; Rowe, R.K.; Kokiko-Cochran, O.N.; Popovich, P.G.; Lifshitz, J.; Eiferman, D.S.; et al. Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation. Glia 2018, 66, 2719–2736. [Google Scholar] [CrossRef] [PubMed]
- Almanza, D.; Gharaee-Kermani, M.; Zhilin-Roth, A.; Rodriguez-Nieves, J.A.; Colaneri, C.; Riley, T.; Macoska, J.A. Nonalcoholic fatty liver disease demonstrates a pre-fibrotic and premalignant molecular signature. Dig. Dis. Sci. 2019, 64, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Lovey, A.; Verma, S.; Kaipilyawar, V.; Ribeiro-Rodrigues, R.; Husain, S.; Palaci, M.; Dietze, R.; Ma, S.; Morrison, R.D.; Sherman, D.R.; et al. Early alveolar macrophage response and IL-1R-dependent T cell priming determine transmissibility of Mycobacterium tuberculosis strains. Nat. Commun. 2022, 13, 884. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Low, J.Z.H.; Gan, E.S.; Kwek, S.S.; Cui, L.; Tan, H.C.; Mok, D.Z.L.; Chan, C.Y.Y.; Sessions, O.M.; Watanabe, S.; et al. Dysregulated metabolism underpins Zika-virus-infection-associated impairment in fetal development. Cell Rep. 2021, 37, 110118. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Mishra, B.; Muzaffar, S.; Gorbatyuk, M.S.; Agarwal, A.; Mukhtar, M.S.; Athar, M. Dynamic regulation of the nexus between stress granules, roquin, and regnase-1 underlies the molecular pathogenesis of warfare vesicants. Front. Immunol. 2021, 12, 809365. [Google Scholar] [CrossRef]
- Elchaninov, A.; Lokhonina, A.; Nikitina, M.; Vishnyakova, P.; Makarov, A.; Arutyunyan, I.; Poltavets, A.; Kananykhina, E.; Kovalchuk, S.; Karpulevich, E.; et al. Comparative analysis of the transcriptome, proteome, and miRNA profile of Kupffer cells and monocytes. Biomedicines 2020, 8, 627. [Google Scholar] [CrossRef]
- Lee, H.N.; McWilliams, I.L.; Lewkowicz, A.P.; Engel, K.; Ireland, D.D.C.; Kelley-Baker, L.; Thacker, S.; Piccardo, P.; Manangeeswaran, M.; Verthelyi, D. Characterization of the therapeutic effect of antibodies targeting the Ebola glycoprotein using a novel BSL2-compliant rVSVDeltaG-EBOV-GP infection model. Emerg. Microbes Infect. 2021, 10, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Lee, H.; Kim, G.Y.; Kim, S.; Cheong, J.; Choi, Y.H. Anti-inflammatory effect of auranofin on palmitic acid and LPS-induced inflammatory response by modulating TLR4 and NOX4-mediated NF-kappaB signaling pathway in RAW264.7 macrophages. Int. J. Mol. Sci. 2021, 22, 5920. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Wang, Y.; Khan, J.; Muzaffar, S.; Lee, M.B.; Weng, Z.; Croutch, C.; Agarwal, A.; Deshane, J.; Athar, M. Role of hair follicles in the pathogenesis of arsenical-induced cutaneous damage. Ann. N. Y. Acad. Sci. 2022, 1515, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, M.P.; Elchaninov, A.V.; Lokhonina, A.V.; Makarov, A.V.; Tagirova, M.K.; Grinberg, M.V.; Bolshakova, G.B.; Glinkina, V.V.; Goldshtein, D.V.; Fatkhudinov, T.K. Analysis of the expression of regulator genes in Kupffer cells and monocytes. Bull. Exp. Biol. Med. 2020, 168, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Syenina, A.; Gan, E.S.; Toh, J.Z.N.; de Alwis, R.; Lin, L.Z.; Tham, C.Y.L.; Yee, J.X.Y.; Leong, S.; Sam, H.; Cheong, C.; et al. Adverse effects following anti-COVID-19 vaccination with mRNA-based BNT162b2 are alleviated by altering the route of administration and correlate with baseline enrichment of T and NK cell genes. PLoS Biol. 2022, 20, e3001643. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Molania, R.; Gagnon-Bartsch, J.A.; Dobrovic, A.; Speed, T.P. A new normalization for Nanostring nCounter gene expression data. Nucleic Acids Res. 2019, 47, 6073–6083. [Google Scholar] [CrossRef]
- Smyth, G.K. Limma: Linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Gentleman, R., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005; pp. 397–420. [Google Scholar]
- Wang, G.; Muschelli, J.; Lindquist, M.A. Moderated t-tests for group-level fMRI analysis. Neuroimage 2021, 237, 118141. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N., Jr. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]





| Gene | Forward Primer | Reverse Primer | Sequence Name |
|---|---|---|---|
| Actb | TCGTGCGTGACATCAAAGAG | GGATTCCATACCCAAGAAGG | β-Actin, cytoskeletal protein (housekeeping gene) |
| Arg1 | TGTCCCTAATGACAGCTCCTT | GCATCCACCCAAATGACACAT | Arginase 1 |
| Ccl2 | GGCTCAGCCAGATGCAGTTAA | CTTGGTGACAAAAACTACAGCTTC | C-C motif chemokine ligand 2 |
| Il1b | TGGCAACTGTTCCTGAACTTC | GGAAGCAGCCCTTCATCTTT | Interleukin 1 beta |
| Il6 | GAAAAGAGTTGTGCAATG | TATGGTACTCCAGAAGAC | Interleukin 6 |
| Il10 | GGACTTTAAGGGTTACTTGG | TCACCCAGGGAATTCAAATG | Interleukin 10 |
| Nfkb1 | GGATGACAGAGGCGTGTATTAG | CCTTCTCTCTGTCTGTGAGTTG | Nuclear factor of kappa light polypeptide gene enhancer in B cells 1, p105 |
| Nlrp3 | GAGCCTACAGTTGGGTGAA | CCTACCAGGAAATCTCGAAGAC | NLR family pyrin domain containing 3 |
| Tnf | CCCTCACACTCAGATCATCT | TGTCTTTGAGATCCATGCCG | Tumor necrosis factor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmond, L.W.; Holbrook, E.M.; Wright, C.T.O.; Zambrano, C.A.; Stamper, C.E.; Bohr, A.D.; Frank, M.G.; Podell, B.K.; Moreno, J.A.; MacDonald, A.S.; et al. Effects of Mycobacterium vaccae NCTC 11659 and Lipopolysaccharide Challenge on Polarization of Murine BV-2 Microglial Cells. Int. J. Mol. Sci. 2024, 25, 474. https://doi.org/10.3390/ijms25010474
Desmond LW, Holbrook EM, Wright CTO, Zambrano CA, Stamper CE, Bohr AD, Frank MG, Podell BK, Moreno JA, MacDonald AS, et al. Effects of Mycobacterium vaccae NCTC 11659 and Lipopolysaccharide Challenge on Polarization of Murine BV-2 Microglial Cells. International Journal of Molecular Sciences. 2024; 25(1):474. https://doi.org/10.3390/ijms25010474
Chicago/Turabian StyleDesmond, Luke W., Evan M. Holbrook, Caelan T. O. Wright, Cristian A. Zambrano, Christopher E. Stamper, Adam D. Bohr, Matthew G. Frank, Brendan K. Podell, Julie A. Moreno, Andrew S. MacDonald, and et al. 2024. "Effects of Mycobacterium vaccae NCTC 11659 and Lipopolysaccharide Challenge on Polarization of Murine BV-2 Microglial Cells" International Journal of Molecular Sciences 25, no. 1: 474. https://doi.org/10.3390/ijms25010474