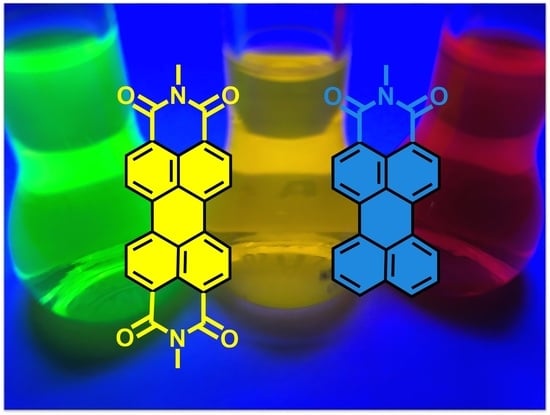
Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy
Abstract
:1. Introduction
2. Presentation and Requirements for Photodynamic Therapy, Photothermal Therapy and Bioimaging Applications
3. PDI and PMI Structures: Common Features and Specificities
3.1. Structural Elements for the Design of PDI and PMI Derivatives
3.2. Perylenediimide (PDI)-Based Building Blocks for Designing New Architectures
3.3. Perylenemonimide (PMI)-Based Building Blocks for Designing Architectures
4. Perylenediimide (PDI)-Based Systems for Bioimaging, PTT and PDT
4.1. Synthesis and Applications of Unsubstituted Bay PDI Materials
4.1.1. Small-Molecule-Based Systems
4.1.2. Polymer-Based Systems
4.2. Synthesis and Applications of Tetrachloro Bay-Substituted PDI Materials
4.2.1. Small-Molecule-Based Systems
4.2.2. Polymer-Based Systems
Grafting the Chain Polymer in the Imide Position
Grafting the Chain Polymer in the Bay Position
4.3. Synthesis and Applications of Dialkoxy and Tetraalkoxy Bay-Substituted PDI Materials
4.3.1. Small-Molecule-Based Systems
4.3.2. Polymer-Based Systems
4.4. Synthesis and Applications of Amino-PDI Bay-Substituted Materials
4.4.1. Small-Molecule-Based Systems
4.4.2. Polymer-Based Systems
Grafting the Chain Polymer in the Imide Position
Grafting the Chain Polymer in the Bay Position
5. Perylenemonoimide (PMI)-Based Systems for Bioimaging and PDT
5.1. Synthesis and Applications of Unsubstituted Bay PMI Materials
5.2. Synthesis and Applications of Substituted Bay and/or Peri PMI Materials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef]
- Borissov, A.; Maurya, Y.K.; Moshniaha, L.; Wong, W.-S.; Żyła-Karwowska, M.; Stępień, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565–788. [Google Scholar] [CrossRef] [PubMed]
- Buess, C.M.; Lawson, D.D. The Preparation, Reactions, and Properties of Triphenylenes. Chem. Rev. 1960, 60, 313–330. [Google Scholar] [CrossRef]
- Sonet, D.; Bibal, B. Triphenylene: A versatile molecular receptor. Tet. Lett. 2019, 60, 872–884. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Zöphel, L.; Enkelmann, V.; Müllen, K. Tuning the HOMO–LUMO Gap of Pyrene Effectively via Donor–Acceptor Substitution: Positions 4,5 Versus 9,10. Org. Lett. 2013, 15, 804–807. [Google Scholar] [CrossRef]
- Chen, Q.; Thoms, S.; Stöttinger, S.; Schollmeyer, D.; Müllen, K.; Narita, A.; Basché, T. Dibenzo[hi,st]ovalene as Highly Luminescent Nanographene: Efficient Synthesis via Photochemical Cyclodehydroiodination, Optoelectronic Properties, and Single-Molecule Spectroscopy. J. Am. Chem. Soc. 2019, 141, 16439–16449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran-Van, A.-F.; Wegner, H.A. Strategies in organic synthesis for condensed arenes, coronene, and graphene. Top. Curr. Chem. 2014, 349, 121–157. [Google Scholar] [CrossRef]
- Kumar, S.; Tao, Y.-T. Coronenes, Benzocoronenes and Beyond: Modern Aspects of Their Syntheses, Properties, and Applications. Chem. Asian J. 2021, 16, 621–647. [Google Scholar] [CrossRef] [PubMed]
- Nestoros, E.; Stuparu, M.C. Corannulene: A molecular bowl of carbon with multifaceted properties and diverse applications. Chem. Commun. 2018, 54, 6503–6519. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, E.M.; Halilovic, D.; Stuparu, M.C. Synthesis of corannulene-based nanographenes. Commun. Chem. 2019, 2, 58. [Google Scholar] [CrossRef] [Green Version]
- Clar, E.; Kelly, W.; Laird, R.M. Die Synthesen des Terrylens und Quaterrylens und über das vermeintliche Quaterrylen von A. Zinke. Mon. Für Chem. 1956, 87, 391–398. [Google Scholar] [CrossRef]
- Biradar, M.R.; Bhosale, S.V.; Morajakar, P.P.; Bhosale, S.V. A review on energy storage devices based on rylene imide dyes: Synthesis, applications and challenges. Fuel 2022, 310, 122487. [Google Scholar] [CrossRef]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and related diimides for organic electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef]
- Liang, N.; Meng, D.; Wang, Z. Giant Rylene Imide-Based Electron Acceptors for Organic Photovoltaics. Acc. Chem. Res. 2021, 54, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Kardos, M. Über einige Aceanthrenchinon- und 1.9-Anthracen-Derivate. Ber. Dtsch. Chem. Ges. 1913, 46, 2085–2091. [Google Scholar] [CrossRef] [Green Version]
- Zollinger, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Herbst, W.; Hunger, K.; Wilker, G.; Ohleier, H.; Winter, R. Industrial Organic Pigments: Production, Properties, Applications, 3rd ed.; Verlag, W.V., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2004. [Google Scholar] [CrossRef]
- Langhals, H. Cyclic Carboxylic Imide Structures as Structure Elements of High Stability. Novel Developments in Perylene Dye Chemistry. Heterocycles 1995, 40, 477–500. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Król, A.; Würthner, F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef] [Green Version]
- Rocard, L.; Goujon, A.; Hudhomme, P. Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials. Molecules 2020, 25, 1402. [Google Scholar] [CrossRef] [Green Version]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-Assembly of Perylene Imide Molecules into 1D Nanostructures: Methods, Morphologies, and Applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Fan, M.; Zheng, X.; Guan, J.; Yin, M. Chirality of Perylene Diimides: Design Strategies and Applications. Angew. Chem. Int. Ed. 2022, 61, e202202532. [Google Scholar] [CrossRef]
- Diacon, A.; Krupka, O.; Hudhomme, P. Fullerene-Perylenediimide (C60-PDI) Based Systems: An Overview and Synthesis of a Versatile Platform for Their Anchor Engineering. Molecules 2022, 27, 6522. [Google Scholar] [CrossRef]
- Sebastian, E.; Hariharan, M. Symmetry-Breaking Charge Separation in Molecular Constructs for Efficient Light Energy Conversion. ACS Energy Lett. 2022, 7, 696–711. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, J.; Kumar, Y.; Mukhopadhyay, P. Electron-poor arylenediimides. Org. Chem. Front. 2018, 5, 2254–2276. [Google Scholar] [CrossRef]
- Schaack, C.; Evans, A.M.; Ng, F.; Steigerwald, M.L.; Nuckolls, C. High-Performance Organic Electronic Materials by Contorting Perylene Diimides. J. Am. Chem. Soc. 2022, 144, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Król, A.; Shoyama, K.; Stolte, M.; Würthner, F. Naphthalene and perylene diimides–better alternatives to fullerenes for organic electronics? Chem. Commun. 2018, 54, 13763–13772. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, G.; Qi, T.; Huang, H. Aromatic imide/amide-based organic small-molecule emitters for organic light-emitting diodes. Mater. Chem. Front. 2020, 4, 1554–1568. [Google Scholar] [CrossRef]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Li, C.; Wonneberger, H. Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dye. Pigm. 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Fernández-Lázaro, F.; Zink-Lorre, N.; Sastre-Santos, Á. Perylenediimides as non-fullerene acceptors in bulk-heterojunction solar cells (BHJSCs). J. Mater. Chem. A 2016, 4, 9336–9346. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, Q.; Gao, X. Non-fullerene small molecule acceptors based on perylene diimides. J. Mater. Chem. A 2016, 4, 17604–17622. [Google Scholar] [CrossRef]
- Macedo, A.G.; Christopholi, L.P.; Gavim, A.E.X.; de Deus, J.F.; Teridi, M.A.M.; Yusoff, A.R.b.M.; da Silva, W.J. Perylene derivatives for solar cells and energy harvesting: A review of materials, challenges and advances. J. Mater. Sci. Mater. Electron. 2019, 30, 15803–15824. [Google Scholar] [CrossRef]
- Fujimoto, K.; Takahashi, M.; Izawa, S.; Hiramoto, M. Development of Perylene-Based Non-Fullerene Acceptors through Bay-Functionalization Strategy. Materials 2020, 13, 2148. [Google Scholar] [CrossRef]
- Zink-Lorre, N.; Font-Sanchis, E.; Sastre-Santos, Á.; Fernández-Lázaro, F. Perylenediimides as more than just non-fullerene acceptors: Versatile components in organic, hybrid and perovskite solar cells. Chem. Commun. 2020, 56, 3824–3838. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, Y.; Sun, C.; Xue, L.; Wang, H.; Zhang, Z.-G. Perylene-diimide derived organic photovoltaic materials. Sci. China Chem. 2022, 65, 462–485. [Google Scholar] [CrossRef]
- Sharma, V.; Koenig, J.D.B.; Welch, G.C. Perylene diimide based non-fullerene acceptors: Top performers and an emerging class featuring N-annulation. J. Mater. Chem. A 2021, 9, 6775–6789. [Google Scholar] [CrossRef]
- Shi, Q.; Wu, J.; Wu, X.; Peng, A.; Huang, H. Perylene Diimide-Based Conjugated Polymers for All-Polymer Solar Cells. Chem. Eur. J. 2020, 26, 12510–12522. [Google Scholar] [CrossRef] [PubMed]
- Soh, N.; Ueda, T. Perylene bisimide as a versatile fluorescent tool for environmental and biological analysis: A review. Talanta 2011, 85, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, G.; Yang, B.; Ji, Q.; Xiang, W.; He, H.; Xu, Z.; Qi, C.; Li, S.; Yang, S.; et al. Review on application of perylene diimide (PDI)-based materials in environment: Pollutant detection and degradation. Sci. Total Environ. 2021, 780, 146483. [Google Scholar] [CrossRef] [PubMed]
- Görl, D.; Zhang, X.; Würthner, F. Molecular Assemblies of Perylene Bisimide Dyes in Water. Angew. Chem. Int. Ed. 2012, 51, 6328–6348. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Tapeh-Esmail, E.; Golshan, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Perylene-3,4,9,10-tetracarboxylic diimide and its derivatives: Synthesis, properties and bioapplications. Dye. Pigm. 2020, 180, 108488. [Google Scholar] [CrossRef]
- Sun, M.; Müllen, K.; Yin, M. Water-soluble perylenediimides: Design concepts and biological applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Xu, Z.; Yin, M. Perylenediimide-cored dendrimers and their bioimaging and gene delivery applications. Prog. Polym. Sci. 2015, 46, 25–54. [Google Scholar] [CrossRef]
- Chen, Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules 2022, 27, 8628. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X. Semiconducting Perylene Diimide Nanostructure: Multifunctional Phototheranostic Nanoplatform. Acc. Chem. Res. 2019, 52, 1245–1254. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, N.; Wang, Y.; Ling, G.; Zhang, P. Perylene diimide-based treatment and diagnosis of diseases. J. Mater. Chem. B 2021, 9, 8937–8950. [Google Scholar] [CrossRef]
- Feiler, L.; Langhals, H.; Polborn, K. Synthesis of perylene-3,4-dicarboximides—Novel highly photostable fluorescent dyes. Liebigs Ann. 1995, 1995, 1229–1244. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Khan, A.; Chatterjee, O.; Bhunia, S.; Apurba, K. Perylene Monoimide as a Versatile Fluoroprobe: The Past, Present, and Future. Org. Mater. 2021, 3, 417–454. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Peng, S.; Tsai, H.-C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Guo, R.; Guo, Y.; Song, J.; Li, Z.; Song, F. Boosting Type-I and Type-II ROS Production of Water-Soluble Porphyrin for Efficient Hypoxic Tumor Therapy. Mol. Pharm. 2023, 20, 606–615. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Konan, Y.N.; Gurny, R.; Allémann, E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B 2002, 66, 89–106. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment-An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, S.; Zhang, F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Rev. Bioeng. 2023, 1, 60–78. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotech. 2009, 4, 710–711. [Google Scholar] [CrossRef] [Green Version]
- Schnermann, M.J. Organic dyes for deep bioimaging. Nature 2017, 551, 176–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.-N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.G.; Adams, S.R.; Ellisman, M.H.; Tsien, R.Y. The Fluorescent Toolbox for Assessing Protein Location and Function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2012, 42, 622–661. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Chen, M.; Yin, M. Design and development of fluorescent nanostructures for bioimaging. Prog. Polym. Sci. 2014, 39, 365–395. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Li, C.; Müllen, K. Beyond perylene diimides: Synthesis, assembly and function of higher rylene chromophores. J. Mater. Chem. C 2014, 2, 1938–1956. [Google Scholar] [CrossRef]
- Ji, C.; Cheng, W.; Yuan, Q.; Müllen, K.; Yin, M. From Dyestuff Chemistry to Cancer Theranostics: The Rise of Rylenecarboximides. Acc. Chem. Res. 2019, 52, 2266–2277. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, A.; Märkle, S.; Langhals, H. Lösliche Perylen-Fluoreszenzfarbstoffe mit hoher Photostabilität. Chem. Ber. 1982, 115, 2927–2934. [Google Scholar] [CrossRef] [Green Version]
- Langhals, H. Synthese von hochreinen Perylen-Fluoreszenzfarbstoffen in großen Mengen–gezielte Darstellung von Atrop-Isomeren. Chem. Ber. 1985, 118, 4641–4645. [Google Scholar] [CrossRef] [Green Version]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, L.J.W.; Rybtchinski, B. Selective bromination of perylene diimides under mild conditions. J. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef]
- Leroy-Lhez, S.; Baffreau, J.; Perrin, L.; Levillain, E.; Allain, M.; Blesa, M.-J.; Hudhomme, P. Tetrathiafulvalene in a Perylene-3,4:9,10-bis(dicarboximide)-Based Dyad: A New Reversible Fluorescence-Redox Dependent Molecular System. J. Org. Chem. 2005, 70, 6313–6320. [Google Scholar] [CrossRef]
- Perrin, L.; Hudhomme, P. Synthesis, Electrochemical and Optical Absorption Properties of New Perylene-3,4:9,10-bis(dicarboximide) and Perylene-3,4:9,10-bis(benzimidazole) Derivatives. Eur. J. Org. Chem. 2011, 2011, 5427–5440. [Google Scholar] [CrossRef] [Green Version]
- Würthner, F.; Stepanenko, V.; Chen, Z.; Saha-Möller, C.R.; Kocher, N.; Stalke, D. Preparation and characterization of regioisomerically pure 1,7-disubstituted perylene bisimide dyes. J. Org. Chem. 2004, 69, 7933–7939. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Chow, T.J. 1,7-Dinitroperylene bisimides: Facile synthesis and characterization as n-type organic semiconductors. Tet. Lett. 2010, 51, 5959–5963. [Google Scholar] [CrossRef]
- Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; et al. High-Performance Solution-Processed Non-Fullerene Organic Solar Cells Based on Selenophene-Containing Perylene Bisimide Acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar] [CrossRef]
- El-Berjawi, R.; Hudhomme, P. Synthesis of a perylenediimide-fullerene C60 dyad: A simple use of a nitro leaving group for a Suzuki-Miyaura coupling reaction. Dye. Pigm. 2018, 159, 551–556. [Google Scholar] [CrossRef]
- Rocard, L.; Hatych, D.; Chartier, T.; Cauchy, T.; Hudhomme, P. Original Suzuki–Miyaura Coupling Using Nitro Derivatives for the Synthesis of Perylenediimide-Based Multimers. Eur. J. Org. Chem. 2019, 2019, 7635–7643. [Google Scholar] [CrossRef]
- Hruzd, M.; Rocard, L.; Goujon, A.; Allain, M.; Cauchy, T.; Hudhomme, P. Desymmetrization of Perylenediimide Bay Regions Using Selective Suzuki–Miyaura Reactions from Dinitro Substituted Derivatives. Chem. Eur. J. 2020, 26, 15881–15891. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, T.; Hiroto, S.; Shinokubo, H. Iridium-Catalyzed Direct Tetraborylation of Perylene Bisimides. Org. Lett. 2011, 13, 2532–2535. [Google Scholar] [CrossRef] [PubMed]
- Battagliarin, G.; Li, C.; Enkelmann, V.; Müllen, K. 2,5,8,11-Tetraboronic Ester Perylenediimides: A Next Generation Building Block for Dye-Stuff Synthesis. Org. Lett. 2011, 13, 3012–3015. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, D.; Zhang, L.; Liu, Y.; Mo, X.; Lin, J.; Zhang, H.-J. Direct Synthesis of Large-Scale Ortho-Iodinated Perylene Diimides: Key Precursors for Functional Dyes. Org. Lett. 2017, 19, 5438–5441. [Google Scholar] [CrossRef]
- Kaiser, H.; Lindner, J.; Langhals, H. Synthese von nichtsymmetrisch substituierten Perylen-Fluoreszenzfarbstoffen. Chem. Ber. 1991, 124, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Langhals, H.; Sprenger, S.; Brandherm, M.-T. Perylenamidine-imide dyes. Liebigs Ann. 1995, 1995, 481–486. [Google Scholar] [CrossRef]
- Wescott, L.D.; Mattern, D.L. Donor−σ−Acceptor Molecules Incorporating a Nonadecyl-Swallowtailed Perylenediimide Acceptor. J. Org. Chem. 2003, 68, 10058–10066. [Google Scholar] [CrossRef]
- Quante, H.; Müllen, K. Quaterrylenebis(dicarboximides). Angew. Chem. Int. Ed. Engl. 1995, 34, 1323–1325. [Google Scholar] [CrossRef]
- Altaş, A.; Gültekin, D.D.; Acar, M.; Cücü, E.; Karatay, A.; Elmalı, A.; Atalay, A.; Demircan, Ç.A.; Bozkaya, U.; Kazaz, C.; et al. Bay- and peri-functionalized donor-acceptor perylene monoimides via nitration and nucleophilic substitution/reduction pathway. Mater. Today Chem. 2022, 24, 100908. [Google Scholar] [CrossRef]
- Zagranyarski, Y.; Chen, L.; Zhao, Y.; Wonneberger, H.; Li, C.; Müllen, K. Facile transformation of perylene tetracarboxylic acid dianhydride into strong donor-acceptor chromophores. Org. Lett. 2012, 14, 5444–5447. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, N.I.; Said, A.I.; Toshkova, R.A.; Tzoneva, R.D.; Bojinov, V.B. A novel water-soluble perylenetetracarboxylic diimide as a fluorescent pH probe: Chemosensing, biocompatibility and cell imaging. Dye. Pigm. 2019, 160, 28–36. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, F.; Zhang, J.; Jiang, T.; Li, X.; Wu, J.; Ren, H. A water-soluble fluorescent pH probe based on perylene dyes and its application to cell imaging. Lumin. J. Biol. Chem. Lumin. 2016, 31, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, M.; Aly, S.; Lant, J.T.; Zhang, X.; Charpentier, P. Energy/Electron Transfer Switch for Controlling Optical Properties of Silicon Quantum Dots. Sci. Rep. 2018, 8, 17068. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Zheng, Y.; Ji, C.; Shen, J.; Yin, M. Self-Assembly and Disassembly of Amphiphilic Zwitterionic Perylenediimide Vesicles for Cell Membrane Imaging. ACS Appl. Mater. Interfaces 2017, 9, 4534–4539. [Google Scholar] [CrossRef]
- Yip, A.M.-H.; Shum, J.; Liu, H.-W.; Zhou, H.; Jia, M.; Niu, N.; Li, Y.; Yu, C.; Lo, K.K.-W. Luminescent Rhenium(I)–Polypyridine Complexes Appended with a Perylene Diimide or Benzoperylene Monoimide Moiety: Photophysics, Intracellular Sensing, and Photocytotoxic Activity. Chem.—A Eur. J. 2019, 25, 8970–8974. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Chou, Y.-T.; Su, B.-K.; Wu, C.-C.; Wang, C.-H.; Chang, K.-H.; Ho, J.-A.A.; Chou, P.-T. Comprehensive Thione-Derived Perylene Diimides and Their Bio-Conjugation for Simultaneous Imaging, Tracking, and Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Jin, X.; Deng, Q.; Yin, Z.; Tong, S.; Qing, W.; Huang, Y. Regioisomer-manipulating thio-perylenediimide nanoagents for photothermal/photodynamic theranostics. J. Mater. Chem. B 2020, 8, 5535–5544. [Google Scholar] [CrossRef]
- Llewellyn, B.A.; Davies, E.S.; Pfeiffer, C.R.; Cooper, M.; Lewis, W.; Champness, N.R. Thionated perylene diimides with intense absorbance in the near-IR. Chem. Commun. 2016, 52, 2099–2102. [Google Scholar] [CrossRef]
- Wang, L.; Sun, C.; Li, S.; Jia, N.; Li, J.; Qu, F.; Goh, K.; Chen, Y. Perylene bisimide-incorporated water-soluble polyurethanes for living cell fluorescence labeling. Polymer 2016, 82, 172–180. [Google Scholar] [CrossRef]
- He, J.; Chen, H.; Guo, Y.; Wang, L.; Zhu, L.; Karahan, H.E.; Chen, Y. Polycondensation of a Perylene Bisimide Derivative and L-Malic Acid as Water-Soluble Conjugates for Fluorescent Labeling of Live Mammalian Cells. Polymers 2018, 10, 559. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, B.; Malhotra, M.; Jayakannan, M. Perylene-Tagged Polycaprolactone Block Copolymers and Their Enzyme-Biodegradable Fluorescent Nanoassemblies for Intracellular Bio-imaging in Cancer Cells. ACS Appl. Polym.Mater. 2019, 1, 3375–3388. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Liu, L.; Lv, F.; Wang, S. Design and Synthesis of Reactive Perylene Tetracarboxylic Diimide Derivatives for Rapid Cell Imaging. ACS Omega 2018, 3, 8691–8696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neelakandan, P.P.; Pan, Z.; Hariharan, M.; Zheng, Y.; Weissman, H.; Rybtchinski, B.; Lewis, F.D. Hydrophobic Self-Assembly of a Perylenediimide-Linked DNA Dumbbell into Supramolecular Polymers. J. Am. Chem. Soc. 2010, 132, 15808–15813. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, C. Fluorescence turn-on detection of a protein through the reduced aggregation of a perylene probe. Angew. Chem. Int. Ed. 2010, 49, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Céspedes-Guirao, F.J.; Ropero, A.B.; Font-Sanchis, E.; Nadal, Á.; Fernández-Lázaro, F.; Sastre-Santos, Á. A water-soluble perylene dye functionalised with a 17β-estradiol: A new fluorescent tool for steroid hormones. Chem. Commun. 2011, 47, 8307–8309. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xu, L.; Neoh, K.G.; Kang, E.-T. Water-soluble highly fluorescent poly[poly(ethylene glycol) methyl ether methacrylate] for cell labeling. J. Mater. Chem. 2011, 21, 6502–6505. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y.; Jiang, R.; Fu, N.; Lu, X.; Tian, C.; Hu, W.; Fan, Q.; Huang, W. Homogeneous near-infrared emissive polymeric nanoparticles based on amphiphilic diblock copolymers with perylene diimide and PEG pendants: Self-assembly behavior and cellular imaging application. Polym. Chem. 2014, 5, 1372–1380. [Google Scholar] [CrossRef]
- Lemouchi, C.; Simonov, S.; Zorina, L.; Gautier, C.; Hudhomme, P.; Batail, P. Amino acid derivatives of perylenediimide and their N–H⋯O peptide bond dipoles-templated solid state assembly into stacks. Org. Biomol. Chem. 2011, 9, 8096–8101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Kuziv, Y.I.; Hudhomme, P.; Krupka, O.M. Dextran-graft-PNIPAM / Au nanoparticles / perylenediimide hybrid system as thermosensitive optical switches and fluorescent labels for potential use in nanophotonics and biomedical applications. Opt. Mater. 2022, 131, 112753. [Google Scholar] [CrossRef]
- Lü, B.; Chen, Y.; Li, P.; Wang, B.; Müllen, K.; Yin, M. Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion. Nat. Commun. 2019, 10, 767. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Chen, Q.; Ma, F.; Huang, Y.; Gao, Y.; Deng, Q.; Qiao, Z.-Y.; Xing, X.; Zhu, J.; et al. Regulating Twisted Skeleton to Construct Organ-Specific Perylene for Intensive Cancer Chemotherapy. Angew. Chem. Int. Ed. 2021, 60, 16215–16223. [Google Scholar] [CrossRef]
- Li, Q.; Hao, X.; Guo, J.; Ren, X.-K.; Xia, S.; Zhang, W.; Feng, Y. Multifunctional Gene Carriers Labeled by Perylene Diimide Derivative as Fluorescent Probe for Tracking Gene Delivery. Macromol. Rapid Commun. 2019, 40, e1800916. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Kim, R.; Prabhakaran, P.; Lee, K.-S. Highly biocompatible amphiphilic perylenediimide derivative for bioimaging. Opt. Mater. Express 2016, 6, 1420. [Google Scholar] [CrossRef]
- Schill, J.; van Dun, S.; Pouderoijen, M.J.; Janssen, H.M.; Milroy, L.-G.; Schenning, A.P.H.J.; Brunsveld, L. Synthesis and Self-Assembly of Bay-Substituted Perylene Diimide Gemini-Type Surfactants as Off-On Fluorescent Probes for Lipid Bilayers. Chem. Eur. J. 2018, 24, 7734–7741. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini surfactants: A new class of self-assembling molecules. J. Am. Chem. Soc. 1993, 115, 10083–10090. [Google Scholar] [CrossRef]
- Sun, M.; Yin, W.; Dong, X.; Yang, W.; Zhao, Y.; Yin, M. Fluorescent supramolecular micelles for imaging-guided cancer therapy. Nanoscale 2016, 8, 5302–5312. [Google Scholar] [CrossRef]
- Cheng, W.; Cheng, H.; Wan, S.; Zhang, X.; Yin, M. Dual-Stimulus-Responsive Fluorescent Supramolecular Prodrug for Antitumor Drug Delivery. Chem. Mater. 2017, 29, 4218–4226. [Google Scholar] [CrossRef]
- Yukruk, F.; Dogan, A.L.; Canpinar, H.; Guc, D.; Akkaya, E.U. Water-Soluble Green Perylenediimide (PDI) Dyes as Potential Sensitizers for Photodynamic Therapy. Org. Lett. 2005, 7, 2885–2887. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, K.; Yang, Z.; Zhang, R.; Yang, M.; Hu, X.; Ma, X.; Bu, L.; Lu, X.; Xiong, X.; et al. Perylene-diimide-based nanoparticles as highly efficient photoacoustic agents for deep brain tumor imaging in living mice. Adv. Mater. 2015, 27, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kaur, S.; Kaur, S.; Bhargava, G.; Kumar, S.; Singh, P. Self-assembled nanofibers of perylene diimide for the detection of hypochlorite in water, bio-fluids and solid-state: Exogenous and endogenous bioimaging of hypochlorite in cells. J. Mater. Chem. B 2019, 8, 125–135. [Google Scholar] [CrossRef]
- Danilov, E.O.; Rachford, A.A.; Goeb, S.; Castellano, F.N. Evolution of the triplet excited state in Pt(II) perylenediimides. J. Phys. Chem. A 2009, 113, 5763–5768. [Google Scholar] [CrossRef] [Green Version]
- Prusakova, V.; McCusker, C.E.; Castellano, F.N. Ligand-Localized Triplet-State Photophysics in a Platinum(II) Terpyridyl Perylenediimideacetylide. Inorg. Chem. 2012, 51, 8589–8598. [Google Scholar] [CrossRef]
- Llewellyn, B.A.; Slater, A.G.; Goretzki, G.; Easun, T.L.; Sun, X.-Z.; Davies, E.S.; Argent, S.P.; Lewis, W.; Beeby, A.; George, M.W.; et al. Photophysics and electrochemistry of a platinum-acetylide disubstituted perylenediimide. Dalton Trans. 2013, 43, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, M.; Steffen, A.; Würthner, F. Near-IR phosphorescent ruthenium(II) and iridium(III) perylene bisimide metal complexes. Angew. Chem. Int. Ed. 2015, 54, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Huang, H.; Rubbiani, R.; Schulze, M.; Würthner, F.; Chao, H.; Gasser, G. Evaluation of Perylene Bisimide-Based RuII and IrIII Complexes as Photosensitizers for Photodynamic Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1745–1752. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Tian, R.; Wu, J.; Fan, Q.; Yung, B.C.; Niu, G.; Jacobson, O.; Wang, Z.; Liu, G.; Yu, G.; et al. Impact of Semiconducting Perylene Diimide Nanoparticle Size on Lymph Node Mapping and Cancer Imaging. ACS Nano 2017, 11, 4247–4255. [Google Scholar] [CrossRef]
- Cui, C.; Yang, Z.; Hu, X.; Wu, J.; Shou, K.; Ma, H.; Jian, C.; Zhao, Y.; Qi, B.; Hu, X.; et al. Organic Semiconducting Nanoparticles as Efficient Photoacoustic Agents for Lightening Early Thrombus and Monitoring Thrombolysis in Living Mice. ACS Nano 2017, 11, 3298–3310. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Z.; Wang, S.; Wang, Z.; Song, J.; Yu, G.; Fan, W.; Dai, Y.; Wang, J.; Shan, L.; et al. Organic Semiconducting Photoacoustic Nanodroplets for Laser-Activatable Ultrasound Imaging and Combinational Cancer Therapy. ACS Nano 2018, 12, 2610–2622. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yuan, P.; Wang, G.; Deng, W.; Tian, S.; Wang, C.; Lu, X.; Huang, W.; Fan, Q. High Density Glycopolymers Functionalized Perylene Diimide Nanoparticles for Tumor-Targeted Photoacoustic Imaging and Enhanced Photothermal Therapy. Biomacromolecules 2017, 18, 3375–3386. [Google Scholar] [CrossRef]
- Sun, P.; Wang, X.; Wang, G.; Deng, W.; Shen, Q.; Jiang, R.; Wang, W.; Fan, Q.; Huang, W. A perylene diimide zwitterionic polymer for photoacoustic imaging guided photothermal/photodynamic synergistic therapy with single near-infrared irradiation. J. Mater. Chem. B 2018, 6, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F.; et al. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Fan, W.; Zou, J.; Tang, W.; Li, L.; He, L.; Shen, Z.; Wang, Z.; Jacobson, O.; Aronova, M.A.; et al. Precision Cancer Theranostic Platform by In Situ Polymerization in Perylene Diimide-Hybridized Hollow Mesoporous Organosilica Nanoparticles. J. Am. Chem. Soc. 2019, 141, 14687–14698. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dai, Y.; Yin, C.; Fan, Q.; Zhang, W.; Song, J.; Yu, G.; Tang, W.; Fan, W.; Yung, B.C.; et al. Activatable Semiconducting Theranostics: Simultaneous Generation and Ratiometric Photoacoustic Imaging of Reactive Oxygen Species In Vivo. Adv. Mater. 2018, 30, 1707509. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Dai, Y.; Chen, J.; Wang, F.; Lin, L.; Liu, Y.; Zhang, F.; Yu, G.; Zhou, Z.; et al. Self-Assembly of Semiconducting-Plasmonic Gold Nanoparticles with Enhanced Optical Property for Photoacoustic Imaging and Photothermal Therapy. Theranostics 2017, 7, 2177–2185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, J.; Wei, J.; Yin, M. Perylenediimide chromophore as an efficient photothermal agent for cancer therapy. Sci. Bull. 2018, 63, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, C.; Hu, Y.; Ji, C.; Li, S.; Yin, M. pH-responsive perylenediimide nanoparticles for cancer trimodality imaging and photothermal therapy. Theranostics 2020, 10, 166–178. [Google Scholar] [CrossRef]
- Aigner, D.; Borisov, S.M.; Petritsch, P.; Klimant, I. Novel near infra-red fluorescent pH sensors based on 1-aminoperylene bisimides covalently grafted onto poly(acryloylmorpholine). Chem. Commun. 2013, 49, 2139–2141. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Tang, W.; Fan, W.; Dai, Y.; Shen, Z.; Lin, L.; Cheng, S.; Liu, Y.; Niu, G.; et al. Stimuli-Responsive Nanotheranostics for Real-Time Monitoring Drug Release by Photoacoustic Imaging. Theranostics 2019, 9, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Makhloutah, A.; Hatych, D.; Chartier, T.; Rocard, L.; Goujon, A.; Felpin, F.-X.; Hudhomme, P. An investigation of palladium-catalyzed Stille-type cross-coupling of nitroarenes in perylenediimide series. Org. Biomol. Chem. 2022, 20, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Leroy-Lhez, S.; Perrin, L.; Baffreau, J.; Hudhomme, P. Perylenediimide derivatives in new donor–acceptor dyads. C. R. Chim. 2006, 9, 240–246. [Google Scholar] [CrossRef]
- Pal, K.; Sharma, V.; Sahoo, D.; Kapuria, N.; Koner, A.L. Large Stokes-shifted NIR-emission from nanospace-induced aggregation of perylenemonoimide-doped polymer nanoparticles: Imaging of folate receptor expression. Chem. Commun. 2018, 54, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Sharma, V.; Koner, A.L. Single-component white-light emission via intramolecular electronic conjugation-truncation with perylenemonoimide. Chem. Commun. 2017, 53, 7909–7912. [Google Scholar] [CrossRef]
- Li, C.; Schöneboom, J.; Liu, Z.; Pschirer, N.G.; Erk, P.; Herrmann, A.; Müllen, K. Rainbow perylene monoimides: Easy control of optical properties. Chemistry 2009, 15, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Chen, X.; Yuan, Z.; Zhang, H.; Luo, G.; Hu, Y.; Chen, Y. Construction of rylene near-infrared absorption dyes with azaperylene monoimide. Dye. Pigment. 2020, 173, 107930. [Google Scholar] [CrossRef]
- Mu, M.; Ke, X.; Cheng, W.; Li, J.; Ji, C.; Yin, M. Perylenemonoimide-Based Colorimetric Probe with High Contrast for Naked-Eye Detection of Fluoride Ions. Anal. Chem. 2022, 94, 11470–11475. [Google Scholar] [CrossRef]
- Busto, N.; García-Calvo, J.; Vicente Cuevas, J.; Herrera, A.; Mergny, J.-L.; Pons, S.; Torroba, T.; García, B. Influence of core extension and side chain nature in targeting G-quadruplex structures with perylene monoimide derivatives. Bioorg. Chem. 2021, 108, 104660. [Google Scholar] [CrossRef]
- Mengji, R.; Acharya, C.; Vangala, V.; Jana, A. A lysosome-specific near-infrared fluorescent probe for in vitro cancer cell detection and non-invasive in vivo imaging. Chem. Commun. 2019, 55, 14182–14185. [Google Scholar] [CrossRef]
- Kaloyanova, S.; Zagranyarski, Y.; Ritz, S.; Hanulová, M.; Koynov, K.; Vonderheit, A.; Müllen, K.; Peneva, K. Water-Soluble NIR-Absorbing Rylene Chromophores for Selective Staining of Cellular Organelles. J. Am. Chem. Soc. 2016, 138, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ni, D.; Cheng, W.; Ji, C.; Wang, Y.; Müllen, K.; Su, Z.; Liu, Y.; Chen, C.; Yin, M. Enzyme-Triggered Disassembly of Perylene Monoimide-based Nanoclusters for Activatable and Deep Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 14014–14018. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, H.; Zhao, L.-X.; Zhang, G.-F.; Hu, Z.; Huang, Z.-L.; Zhu, M.-Q. A trident dithienylethene-perylenemonoimide dyad with super fluorescence switching speed and ratio. Nat. Commun. 2014, 5, 5709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-X.; Xin, B.; Li, C.; Gong, W.-L.; Huang, Z.-L.; Tang, B.-Z.; Zhu, M.-Q. Photoswitchable polyfluorophores based on perylenemonoimide–dithienylethene conjugates as super-resolution MitoTrackers. J. Mater. Chem. C 2017, 5, 9339–9344. [Google Scholar] [CrossRef]
- Liu, J.-X.; Xin, B.; Li, C.; Xie, N.-H.; Gong, W.-L.; Huang, Z.-L.; Zhu, M.-Q. PEGylated Perylenemonoimide-Dithienylethene for Super-Resolution Imaging of Liposomes. ACS Appl. Mater. Interfaces 2017, 9, 10338–10343. [Google Scholar] [CrossRef] [PubMed]





















































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupka, O.; Hudhomme, P. Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 6308. https://doi.org/10.3390/ijms24076308
Krupka O, Hudhomme P. Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy. International Journal of Molecular Sciences. 2023; 24(7):6308. https://doi.org/10.3390/ijms24076308
Chicago/Turabian StyleKrupka, Oksana, and Piétrick Hudhomme. 2023. "Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy" International Journal of Molecular Sciences 24, no. 7: 6308. https://doi.org/10.3390/ijms24076308