Discovery of 1,2,4-Oxadiazole Derivatives Containing Haloalkyl as Potential Acetylcholine Receptor Nematicides
Abstract
:1. Introduction
2. Result and Discussion
2.1. Chemistry
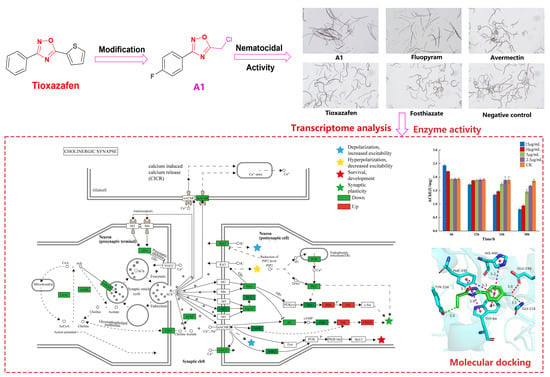
2.2. Nematocidal Activity
2.3. DEGs Analysis
2.4. qRT-PCR Validation
2.5. Enzymatic Activity of AChE
2.6. Molecular Docking and MD Simulations
2.6.1. Molecular Docking
2.6.2. MD Simulations
3. Materials and Methods
3.1. Instruments and Chemicals
3.1.1. General Procedure for the Synthesis of Intermediate 1
3.1.2. General Procedure for the Synthesis Compounds A1–A29 and B1–B19
3.2. Nematocidal Activity Assay
3.3. Transcriptome Profiling
3.4. Quantitative Real-Time Polymerase Chain Reaction
3.5. Enzyme Activity of AChE
3.6. Molecular Docking and MD Simulations
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caboni, P.; Aissani, N.; Cabras, T.; Falqui, A.; Marotta, R.; Liori, B.; Ntalli, N.; Sarais, G.; Sasanelli, N.; Tocco, G. Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 1794–1803. [Google Scholar] [CrossRef]
- Che, Z.; Guo, X.; Li, Y.; Zhang, S.; Zhu, L.; He, J.; Sun, D.; Guo, Y.; Liu, Y.; Wei, R.; et al. Synthesis of paeonol ester derivatives and their insecticidal, nematicidal, and anti-oomycete activities. Pest Manag. Sci. 2022, 78, 3442–3455. [Google Scholar] [CrossRef]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, H.Y.; Wang, S.; Cai, Q.F.; Zhang, Y.; Zou, Y.; Liu, X.; Chen, J.X. Discovery of quinazoline compound as a novel nematicidal scaffold. Pestic. Biochem. Physiol. 2022, 189, 105310. [Google Scholar] [CrossRef]
- Hsu, J.K.; Weng, C.W.; Chen, J.J.; Chen, P.J. The ACE genes in Aphelenchoides besseyi isolates and their expression correlation to the fenamiphos treatment. Sci. Rep. 2022, 12, 1975. [Google Scholar] [CrossRef]
- Jeger, M.; Bragard, C.; Caffier, D. Risk to plant health of Ditylenchus destructor for the EU territory. EFSA J. 2016, 14, e04602. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.Y.; Xu, S.; Wu, J.; Luo, J.Y.; Duan, Y.B.; Wang, J.X.; Zhang, F.; Zhou, M.G. Screening and characterization of Xanthomonasoryzae pv. oryzae strains with resistance to pheazine-1-carboxylic acid. Pestic. Biochem. Phys. 2018, 145, 8–14. [Google Scholar] [CrossRef]
- Kumari, S. Morphological and molecular characterizations of cereal cyst nematode Heterodera avenae Wollenweber, 1924 from the Czech Republic. J. Integr. Agric. 2017, 16, 532–539. [Google Scholar] [CrossRef]
- Whorton, M.D.; Foliart, D.E. Mutagenicity, carcinogenicity and reproductive effects of dibromochloropropane (DBCP). Mutat. Res. Rev. Genet. Toxicol. 1983, 123, 13–30. [Google Scholar] [CrossRef]
- Vanegas, J.A.G.; Pacule, H.B.; Capitão, R.M.; Correia, C.R.D.; Terra, C.W.; Campos, V.P.; Oliveirae, D.F. Methyl esters of (E)-cinnamic acid: Activity against the plant-parasitic nematode Meloidogyne incognita and in silico interaction with histone deacetylase. J. Agric. Food Chem. 2022, 70, 6624–6633. [Google Scholar] [CrossRef]
- Fuller, V.L.; Lilley, C.J.; Urwin, P.E. Nematode resistance. New Phytol. 2008, 180, 27–44. [Google Scholar] [CrossRef]
- Faizi, S.; Fayyaz, S.; Bano, S.; Iqbal, E.Y.; Siddiqi, H.; Naz, A. Isolation of nematicidal compounds from Tagetes patula L. yellow flowers: Structure−activity relationship studies against cyst nematode Heterodera zeae infective stage larvae. J. Agric. Food Chem. 2011, 59, 9080–9093. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E,E)-2,4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef]
- Caboni, P.; Sarais, G.; Aissani, N.; Tocco, G.; Sasanelli, N.; Liori, B.; Carta, A.; Angioni, A. Nematicidal activity of 2-thiophenecarboxaldehyde and methylisothiocyanate from caper (Capparis spinosa) against Meloidogyne incognita. J. Agric. Food Chem. 2012, 60, 7345–7351. [Google Scholar] [CrossRef]
- Bondi, A.V. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Pitzer, K.S. The nature of the chemical bond and the structure of molecules and crystals: An introduction to modern structural chemistry. J. Am. Chem. Soc. 1960, 82, 441–451. [Google Scholar] [CrossRef]
- Lien, E.J.; Guo, Z.R.; Li, R.L.; Su, C.T. Use of dipole moment as a parameter in drug-receptor interaction and quantitative structure-activity relationship studies. J. Pharm. Sci. 1982, 71, 641–655. [Google Scholar] [CrossRef]
- Corradi, E.; Meille, S.V.; Messina, M.T.; Messina, M.T.; Metrangolo, P.; Resnati, G. Halogen bonding versus hydrogen bonding in driving self-assembly processes. Angew. Chem. Int. Ed. 2000, 39, 1782–1786. [Google Scholar] [CrossRef]
- Jeschke, P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag. Sci. 2010, 66, 10–27. [Google Scholar] [CrossRef]
- Jeschke, P. Latest generation of halogen-containing pesticides. Pest Manag. Sci. 2017, 73, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Lommerse, J.P.M.; Price, S.L.; Taylor, R. Hydrogen bonding of carbonyl, ether, and ester oxygen atoms with alkanol hydroxyl groups. J. Comput. Chem. 1997, 18, 757–774. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Substituent constants for correlation analysis in chemistry and biology. Wiley 1979, 69, 1109. [Google Scholar] [CrossRef]
- Devendar, P.; Yang, G.F. Sulfur-containing agrochemicals. Top Curr. Chem. 2017, 375, 82. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Luo, L.; Wang, Z.X.; Ma, X.Y.; Gan, X.H. Design, synthesis and antifungal/nematicidal activity of novel 1,2,4-oxadiazole derivatives containing amide fragments. Int. J. Mol. Sci. 2022, 23, 1596. [Google Scholar] [CrossRef]
- Yang, S.; Ren, C.L.; Ma, T.Y.; Zou, W.Q.; Dai, L.; Tian, X.Y.; Liu, X.H.; Tan, C.X. 1,2,4-Oxadiazole-based bio-isosteres of benzamides: Synthesis, biological activity and toxicity to zebrafish embryo. Int. J. Mol. Sci. 2021, 22, 2367. [Google Scholar] [CrossRef]
- Hang, T.H.; Chen, H.; Chen, J.; Zhang, A.D. Syntheses, crystal structures, and biological activities of two 5-pyrimidinyl-1,2,4-oxadiazoles. Chin. J. Struc. Chem. 2014, 10, 1455–1459. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, Q.Q.; Zhang, J.; Song, J.L.; Li, J.C.; Han, K.; Huang, J.T.; Jiang, C.S.; Zhang, H. Synthesis and evaluation of 1,2,4-oxadiazole derivatives as potential anti-inflammatory agents by inhibiting NF-κB signaling pathway in LPS-stimulated RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2020, 30, 127373. [Google Scholar] [CrossRef]
- Battisti, A.; Piccionello, A.P.; Sgarbossa, A.; Vilasi, S.; Ricci, C.; Ghetti, F.; Spinozzi, F.; Gammazza, A.M.; Giacalone, V.; Martorana, A.; et al. Curcumin-like compounds designed to modify amyloid beta peptide aggregation patterns. RSC Adv. 2017, 7, 31714–31724. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos Filho, J.M.; Macedo, T.S.; Teixeira, H.M.P.; Moreira, D.R.M.; Challal, S.; Wolfender, J.L.; Queiroz, E.F.; Soares, M.B.P. Conjugation of N-acylhydrazone and 1,2,4-oxadiazole leads to the identification of active antimalarial agents. Bioorg. Med. Chem. 2016, 24, 5693–5701. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, R.; Gao, S.; Ma, S.H.; Tang, H.J.; Yang, J.J.; Diao, Y.M.; Wang, H.L.; Zhu, H.J. Structure-based bioisosterism design, synthesis, insecticidal activity and structure-activity relationship (SAR) of anthranilic diamide analogues containing 1,2,4-oxadiazole ring. Pest Manag. Sci. 2017, 73, 917–924. [Google Scholar] [CrossRef]
- Slomczynska, U.; South, M.S.; Bunkers, G.J.; Edgecomb, D.; Wyse-Pester, D.; Selness, S.; Ding, Y.W.; Christiansen, J.; Ediger, K.; Miller, W.; et al. Tioxazafen: A new broad-spectrum seed treatment nematicide. J. Am. Chem. Soc. 2015, 10, 129–147. [Google Scholar] [CrossRef]
- Jeschke, P. Progress of modern agricultural chemistry and future prospects. Pest Manag. Sci. 2016, 72, 433–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.X.; Li, Q.X.; Song, B.A. Chemical nematicides: Recent research progress and outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef]
- Luo, L.; Liu, D.; Lan, S.C.; Gan, X.H. Design, synthesis, and biological activity of novel chalcone derivatives containing 1,2,4-oxadiazole moiety. Front. Chem. 2022, 10, 943062. [Google Scholar] [CrossRef]
- Mayer, J.C.P.; Sauer, A.C.; Iglesias, B.A.; Acunha, T.V.; Back, D.F.; Rodrigues, O.E.D.; Dornelles, L. Ferrocenylethenyl-substituted 1,3,4-oxadiazolyl-1,2,4-oxadiazoles: Synthesis, characterization and DNA-binding assays. J. Org. Chem. 2017, 841, 1–11. [Google Scholar] [CrossRef]
- Mashimo, M.; Moriwaki, Y.; Misawa, H.; Kawashima, K.; Fujii, T. Regulation of immune functions by non-neuronal acetylcholine (ACh) via muscarinic and nicotinic ACh receptors. Int. J. Mol. Sci. 2021, 22, 6818. [Google Scholar] [CrossRef]
- Oda, Y.; Nakanishi, I.; Deguchi, T. A complementary DNA for human choline acetyltransferase induces two forms of enzyme with different molecular weights in cultured cells. Mol. Brain Res. 1992, 16, 287–294. [Google Scholar] [CrossRef]
- Eiden, L.E.; Schäfer, M.K.H.; Weihe, E.; Schütz, B. The vesicular amine transporter family (SLC18): Amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflug. Arch. Eur. J. Phy. 2004, 447, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Başkurt, S.; Taşkin, B.G.; Doğaç, E.; Taşkın, V. Polymorphism in the acetylcholinesterase gene of Musca domestica L. field populations in Turkey. J. Vector Ecol. 2011, 36, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Temeyer, K.B.; Olafson, P.U.; Brake, D.K.; Tuckow, A.P.; Li, A.Y.; de León, A.A.P. Acetylcholinesterase of Rhipicephalus (Boophilus) microplus and Phlebotomus papatasi: Gene identification, expression, and biochemical properties of recombinant proteins. Pestic. Biochem. Physiol. 2013, 106, 118–123. [Google Scholar] [CrossRef]
- Wei, C.Q.; Huang, J.J.; Luo, Y.Q.; Wang, S.B.; Wu, S.K.; Xing, Z.F.; Chen, J.X. Novel amide derivatives containing an imidazo [1,2-a] pyridine moiety: Design, synthesis as potential nematicidal and antibacterial agents. Pestic. Biochem. Phys. 2021, 175, 104857. [Google Scholar] [CrossRef]
- Liu, L.; Alam, M.S.; Hirata, K.; Matsuda, K.; Ozoe, Y. Actions of quinolizidine alkaloids on Periplaneta americana nicotinic acetylcholine receptors. Pest Manag. Sci. 2008, 64, 1222–1228. [Google Scholar] [CrossRef]
- Kalb, A.; von Haefen, C.; Sifringer, M.; Tegethoff, A.; Paeschke, N.; Kostova, M.; Feldheiser, A.; Spies, C.D. Acetylcholinesterase inhibitors reduce neuroinflammation and-degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS ONE 2013, 8, e62679. [Google Scholar] [CrossRef]
- Tao, Q.Q.; Liu, L.W.; Wang, P.Y.; Long, Q.S.; Zhao, Y.L.; Jin, L.H.; Xu, W.M.; Chen, Y.; Li, Z.; Yang, S. Synthesis and in vitro and in vivo biological activity evaluation and quantitative proteome profiling of oxadiazoles bearing flexible heterocyclic patterns. J. Agric. Food Chem. 2019, 67, 7626–7639. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.W.; Liu, N.N.; Zhou, S.; Zhang, L.L.; Yin, H.; Wang, G.Q.; Fan, Z.J.; Ma, Y. Design, synthesis, and biological activity of novel aromatic amide derivatives containing sulfide and sulfone substructures. Engineering 2020, 6, 553–559. [Google Scholar] [CrossRef]
- Ge, H.; Lin, K.; Zhou, C.; Lin, Q.; Zhang, Z.; Wu, J.; Zheng, L.; Yang, Q.; Wu, S.; Chen, W.; et al. A multi-omic analysis of orange-spotted grouper larvae infected with nervous necrosis virus identifies increased adhesion molecules and collagen synthesis in the persistent state. Fish Shellfish Immunol. 2020, 98, 595–604. [Google Scholar] [CrossRef]
- Guo, M.X.; Liu, J.; Xu, Z.L.; Wang, J.; Li, T.T.; Duan, X.W.; Sun, Y.M.; Zhang, X.Y.; Huang, R.M. 2-Methoxy-1,4-naphthoquinone induces metabolic shifts in Penicillium digitatum revealed by high-dimensional biological data. J. Agric. Food Chem. 2020, 68, 9697–9706. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hao, X.; Wang, B.; Ma, L. Transcriptomics and coexpression network profiling of the effects of levamisole hydrochloride on Bursaphelenchus xylophilus. Pestic. Biochem. Physiol. 2022, 181, 105019. [Google Scholar] [CrossRef] [PubMed]











| Compound | B. xylophilus | A. besseyi | D. dipsaci | ||||
|---|---|---|---|---|---|---|---|
| 50 μg/mL | 10 μg/mL | LC50 μg/mL | 50 μg/mL | 10 μg/mL | 50 μg/mL | 10 μg/mL | |
| A1 | 100 | 95.3 ± 2.8 | 2.4 ± 0.1 | 100 | 87.1 ± 4.8 | 100 | 91.2 ± 3.3 |
| A2 | 100 | 90.0 ± 2.6 | 2.8 ± 0.1 | 100 | 60.5 ± 1.9 | 100 | 81.0 ± 1.6 |
| A3 | 100 | 85.3 ± 5.9 | 3.3 ± 0.2 | 100 | 57.2 ± 9.1 | 100 | 80.4 ± 5.8 |
| A4 | 100 | 100 | 4.2 ± 0.4 | 100 | 40.7 ± 4.6 | 100 | 92.8 ± 0.8 |
| A5 | 100 | 63.2±8.1 | 5.0 ± 0.2 | 100 | 62.1 ± 7.1 | 100 | 80.5 ± 4.5 |
| A6 | 100 | 73.4 ± 9.1 | 5.4 ± 0.2 | 100 | 100 | 100 | 97.4 ± 0.7 |
| A7 | 100 | 100 | 5.5 ± 0.3 | 100 | 100 | 100 | 90.2 ± 3.0 |
| A8 | 100 | 66.9 ± 8.6 | 6.5 ± 0.2 | 100 | 49.7 ± 9.8 | 94.8 ± 1.8 | 67.8 ± 4.2 |
| A9 | 100 | 91.7 ± 9.0 | 6.6 ± 1.1 | 100 | 29.5 ± 8.3 | 100 | 90.5 ± 1.2 |
| A10 | 100 | 75.0 ± 1.2 | 6.6 ± 1.2 | 100 | 23.9 ± 1.7 | 100 | 83.9 ± 3.5 |
| A11 | 100 | 89.1 ± 6.2 | 7.0 ± 1.3 | 100 | 36.5 ± 2.4 | 100 | 87.9 ± 6.4 |
| A12 | 100 | 67.0 ± 5.8 | 8.2 ± 0.3 | 100 | 47.7 ± 2.5 | 100 | 74.9 ± 4.2 |
| A13 | 100 | 87.2 ± 1.0 | 8.6 ± 5.3 | 100 | 28.5 ± 2.0 | 100 | 70.6 ± 5.6 |
| A14 | 100 | 58.7 ± 4.2 | 8.9 ± 1.3 | 100 | 65.0 ± 0.4 | 100 | 91.7 ± 4.3 |
| A15 | 100 | 40.3 ± 1.7 | 11.2 ± 0.6 | 100 | 54.3 ± 7.5 | 100 | 67.1 ± 4.9 |
| A16 | 87.0 ± 6.8 | 36.3 ± 3.9 | 13.4 ± 0.7 | 59.4 ± 1.0 | 17.1 ± 8.6 | 100 | 35.5 ± 7.9 |
| A17 | 100 | 20.7 ± 3.3 | 24.9 ± 1.5 | 100 | 26.6 ± 3.8 | 100 | 26.2 ± 1.8 |
| A18 | 100 | 21.8 ± 7.6 | 23.7 ± 2.5 | 90.6 ± 9.4 | 23.4 ± 3.0 | 100 | 43.8 ± 3.8 |
| A19 | 80.6 ± 4.6 | 24.4 ± 4.3 | 26.6 ± 3.7 | 63.5 ± 9.0 | 11.8 ± 5.4 | 100 | 62.1 ± 7.6 |
| A20 | 80.3 ± 4.4 | 27.5 ± 5.6 | 26.7 ± 0.5 | 70.5 ± 4.2 | 31.3 ± 9.2 | 77.4 ± 3.9 | 56.3 ± 1.9 |
| A21 | 95.9 ± 0.7 | 36.1 ± 3.5 | 29.8 ± 0.9 | 32.0 ± 5.1 | 0 | 62.2 ± 4.1 | 0 |
| A22 | 72.7±7.5 | 19.3 ± 3.1 | 36.1 ± 1.0 | 15.0 ± 6.0 | 0 | 78.1 ± 4.3 | 23.3 ± 8.8 |
| A23 | 69.0 ± 8.4 | 0 | 39.7 ± 2.4 | 0 | 0 | 82.1 ± 1.5 | 18.8 ± 6.5 |
| A24 | 45.2 ± 0.3 | 0 | 58.2 ± 6.9 | 0 | 0 | 61.5 ± 1.8 | 21.7 ± 9.7 |
| A25 | 23.2 ± 6.3 | 0 | 89.9 ± 1.6 | 0 | 0 | 33.3 ± 5.1 | 0 |
| A26 | 0 | 0 | 133.4 ± 5.9 | 0 | 0 | 25.2 ± 8.9 | 0 |
| A27 | 0 | 0 | 156.7 ± 9.8 | 0 | 0 | 29.1 ± 6.0 | 16.9 ± 5.6 |
| A28 | 0 | 0 | 203.3 ± 7.2 | 0 | 0 | 22.4 ± 8.6 | 0 |
| A29 | - | - | 216.4 ± 7.9 | - | - | 43.8 ± 7.6 | 19.8 ± 2.8 |
| B1 | 100 | 100 | 2.6 ± 0.7 | 100 | 89.9 ± 2.4 | 100 | 100 |
| B2 | 100 | 100 | 3.5 ± 0.1 | 100 | 100 | 100 | 90.1 ± 5.5 |
| B3 | 100 | 75.2 ± 8.1 | 3.5 ± 0.4 | 100 | 89.9 ± 2.0 | 100 | 86.3 ± 2.5 |
| B4 | 100 | 78.2 ± 1.5 | 3.7 ± 0.3 | 100 | 100 | 100 | 86.9 ± 5.1 |
| B5 | 100 | 100 | 3.8 ± 0.3 | 100 | 100 | 100 | 85.2 ± 7.0 |
| B6 | 100 | 97.4 ± 4.4 | 4.5 ± 0.3 | 100 | 85.8 ± 3.2 | 100 | 87.0 ± 1.1 |
| B7 | 100 | 80.1 ± 3.4 | 4.8 ± 0.2 | 100 | 72.0 ± 4.2 | 100 | 71.9 ± 3.8 |
| B8 | 100 | 93.6 ± 3.9 | 4.9 ± 0.1 | 100 | 100 | 100 | 80.3 ± 3.6 |
| B9 | 100 | 80.8 ± 4.6 | 6.9 ± 6.7 | 100 | 19.5 ± 1.5 | 100 | 88.2 ± 6.8 |
| B10 | 100 | 58.3 ± 3.3 | 8.5 ± 0.5 | 100 | 100 | 100 | 66.7 ± 7.7 |
| B11 | 100 | 75.5 ± 6.3 | 9.5 ± 0.8 | 100 | 30.7 ± 5.3 | 100 | 86.0 ± 5.8 |
| B12 | 100 | 31.5 ± 0.8 | 13.3 ± 0.5 | 20.6 ± 6.6 | 0 | 94.6 ± 5.3 | 37.0 ± 9.6 |
| B13 | 100 | 30.0 ± 4.4 | 14.3 ± 0.5 | 19.8 ± 9.7 | 7.3 ± 1.7 | 100 | 40.6 ± 9.1 |
| B14 | 97.3 ± 2.5 | 0 | 15.2 ± 0.2 | 18.4 ± 4.3 | 0 | 68.2 ± 7.9 | 20.5 ± 2.4 |
| B15 | 76.1 ± 5.0 | 0 | 31.9 ± 6.0 | 28.4 ± 7.7 | 0 | 67.2 ± 2.6 | 23.4 ± 4.7 |
| B16 | 35.0 ± 3.6 | 0 | 60.6 ± 9.0 | 100 | 88.4 ± 2.3 | 51.3 ± 9.6 | 0 |
| B17 | 38.9 ± 5.9 | 0 | 67.6 ± 5.8 | 30.5 ± 9.3 | 0 | 31.4 ± 3.6 | 22.7 ± 5.2 |
| B18 | 0 | 0 | 158.5 ± 8.2 | 0 | 0 | 54.2 ± 3.3 | 23.4 ± 9.3 |
| B19 | 0 | 0 | 200.0 ± 9.1 | 0 | 0 | 34.7 ± 4.7 | 0 |
| Control b | 0 | 0 | 436.9 ± 3.8 | 10.2 ± 1.4 | 0 | 13.3 ± 9.4 | 0 |
| Control c | 0 | 0 | 335.5 ± 3.8 | 42.4 ± 2.1 | 22.2 ± 3.2 | 16.2 ± 4.4 | 0 |
| Control d | 100 | 100 | 0.9 ± 0.07 | 100 | 69.7 ± 1.6 | 100 | 100 |
| Control e | 0 | 0 | >300 | 22.1 ± 2.6 | 0 | 29.0 ± 3.8 | 0 |
| Compound | A. besseyi | D. dipsaci | ||||
|---|---|---|---|---|---|---|
| LC50 μg/mL | Regression Equation | R | LC50 μg/mL | Regression Equation | R | |
| A1 | 6.4 ± 0.6 | Y = 3.9x + 1.8 | 0.95 | 3.6 ± 1.6 | Y = 5.6x + 1.9 | 0.96 |
| A2 | 5.0 ± 0.3 | Y = 2.8x + 3.0 | 0.95 | 3.9 ± 0.4 | Y = 3.7x + 2.8 | 0.99 |
| A3 | 9.6 ± 4.5 | Y = 2.1x + 3.0 | 0.95 | 4.6 ± 0.1 | Y = 2.1x + 3.6 | 0.97 |
| A4 | 11.3±6.3 | Y = 4.5x + 0.3 | 0.96 | 3.5 ± 0.9 | Y = 2.9x + 3.4 | 0.94 |
| A6 | 3.8 ± 1.7 | Y = 2.0x + 3.8 | 0.90 | 4.0 ± 0.4 | Y = 4.0x + 2.6 | 0.93 |
| A7 | 4.8 ± 0.4 | Y = 2.5x + 3.3 | 0.90 | 2.7 ± 0.1 | Y = 7.3x + 1.6 | 0.95 |
| A9 | 13.6 ± 0.3 | Y = 2.6x + 2.1 | 0.96 | 4.4 ± 0.2 | Y = 3.6x + 2.7 | 0.97 |
| A10 | 20.1 ± 1.6 | Y = 4.1x − 0.3 | 0.93 | 3.6 ± 2.5 | Y = 6.6x + 1.3 | 0.95 |
| A11 | 15.3 ± 0.6 | Y = 4.9x − 0.8 | 0.94 | 7.0 ± 1.7 | Y = 2.8x + 2.6 | 0.90 |
| A13 | 16.5 ± 2.4 | Y = 2.5x + 1.9 | 0.94 | 7.2 ± 0.9 | Y = 3.0x + 2.4 | 0.92 |
| A23 | 12.3 ± 4.8 | Y = 2.3x + 2.5 | 0.97 | 10.7 ± 0.6 | Y = 2.3x + 2.6 | 0.96 |
| B1 | 5.1 ± 2.1 | Y = 4.1x + 2.1 | 0.91 | 4.5 ± 0.3 | Y = 3.4x + 2.8 | 0.98 |
| B2 | 6.7 ± 0.2 | Y = 1.8x + 3.5 | 0.91 | 4.6 ± 0.6 | Y = 3.6x + 2.6 | 0.96 |
| B3 | 7.2 ± 2.5 | Y = 2.9x + 2.5 | 0.96 | 3.2 ± 1.4 | Y = 7.9x + 4.1 | 0.99 |
| B4 | 6.4±0.7 | Y = 2.7x + 2.8 | 0.92 | 3.7 ± 2.3 | Y = 4.6x + 2.4 | 0.95 |
| B5 | 5.1 ± 0.1 | Y = 3.5x + 2.5 | 0.93 | 3.7 ± 2.1 | Y = 6.7x + 1.2 | 0.94 |
| B6 | 8.7 ± 2.7 | Y = 3.8x + 1.4 | 0.97 | 4.2 ± 0.1 | Y = 5.7x + 1.4 | 0.94 |
| B8 | 6.2 ± 1.0 | Y = 2.7x + 2.9 | 0.93 | 3.0 ± 0.2 | Y = 4.5x + 2.9 | 0.91 |
| B9 | 14.5 ± 1.9 | Y = 1.2x + 3.6 | 0.93 | 4.2 ± 6.0 | Y = 4.3x + 2.3 | 0.92 |
| B11 | 14.2 ± 3.8 | Y = 3.3x + 1.2 | 0.98 | 3.7 ± 0.1 | Y = 7.2x + 0.9 | 0.98 |
| Control b | 388.5 ± 6.0 | Y = 4.8x − 7.4 | 0.94 | 333.3 ± 9.5 | Y = 5.1x − 7.9 | 0.91 |
| Control c | 56.8 ± 5.2 | Y = 3.5x − 1.1 | 0.98 | 285.4 ± 5.4 | Y = 4.9x − 7.0 | 0.90 |
| Control d | 1.5 ± 0.1 | Y = 2.5x + 4.5 | 0.96 | 0.8 ± 0.1 | Y = 2.8x + 5.3 | 0.92 |
| Control e | 142.9 ± 7.4 | Y = 3.5x − 2.5 | 0.94 | >300 | - | - |
| Compounds | ΔEvdw | ΔEele | ΔEMM | ΔGsol | ΔEbind | −TΔS | ΔGbind |
|---|---|---|---|---|---|---|---|
| A1 | −141.47 | −14.19 | −155.66 | 111.69 | −43.97 | 5.89 | −38.08 |
| Fosthiazate | −103.39 | −43.35 | −146.74 | 88.02 | −58.72 | 31.25 | −27.47 |
| Genes | Sequences (5′–3′) |
|---|---|
| AChE 1-F | ACCGGCACTTTCACCGAATA |
| AChE 1-R | AGCCCCAGGGAATATGGGAT |
| AChE 2-F | TGTCCGACATGCTTTGCTTA |
| AChE 2-R | ATCCCCAACAAATCGGGCAA |
| ChAT-F | TGCGACACTGTGTTCAATGC |
| ChAT-R | CGGAAGCAATAATGCAGGGC |
| Actin-F | GAAAGAGGGCCGGAAGAG |
| Actin-R | AGATCGTCCGCGACATAAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Ou, Y.; Zhang, Q.; Gan, X. Discovery of 1,2,4-Oxadiazole Derivatives Containing Haloalkyl as Potential Acetylcholine Receptor Nematicides. Int. J. Mol. Sci. 2023, 24, 5773. https://doi.org/10.3390/ijms24065773
Luo L, Ou Y, Zhang Q, Gan X. Discovery of 1,2,4-Oxadiazole Derivatives Containing Haloalkyl as Potential Acetylcholine Receptor Nematicides. International Journal of Molecular Sciences. 2023; 24(6):5773. https://doi.org/10.3390/ijms24065773
Chicago/Turabian StyleLuo, Ling, Yuqin Ou, Qi Zhang, and Xiuhai Gan. 2023. "Discovery of 1,2,4-Oxadiazole Derivatives Containing Haloalkyl as Potential Acetylcholine Receptor Nematicides" International Journal of Molecular Sciences 24, no. 6: 5773. https://doi.org/10.3390/ijms24065773