Cytotoxic Effects of Darinaparsin, a Novel Organic Arsenical, against Human Leukemia Cells
Abstract
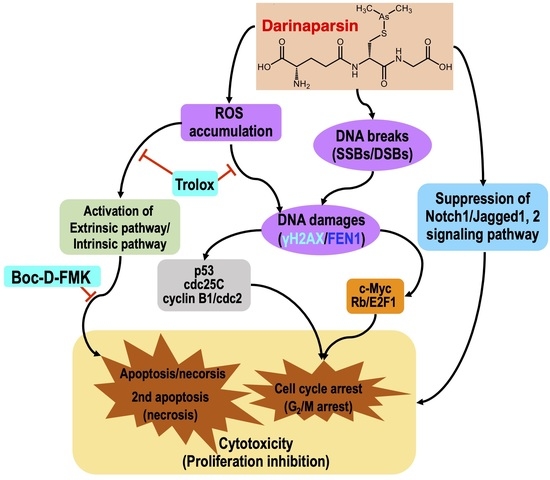
:1. Introduction
2. Results
2.1. More Potent Cytotoxicity Induced by Darinaparsin in Comparison with AsIII in Human Leukemia Cell Lines
2.2. Contribution of Apoptosis and Necrosis to the Cytocidal Effect of Darinaparsin against NB4 and HL-60 Cells
2.3. Darinaparsin-Triggered Caspase Activation and Bid Truncation in NB4 Cells
2.4. Suppression of Darinaparsin-Mediated Cell Death by Boc-D-FMK in NB4 Cells
2.5. Suppression of Darinaparsin-Mediated Cell Death by Trolox in NB4 Cells
2.6. Effect of Darinaparsin on the Cell Cycle Profiling and the Expression Level of Cell Cycle Related-Gene Proteins in NB4 Cells
2.7. Effect of Trolox on G2/M Phase Arrest in NB4 Cells Treated with Darinaparsin
2.8. Effect of Darinaparsin on Notch1 Signaling-Related Gene Protein Expression in NB4 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Determination of Apoptosis
4.4.1. Annexin V/PI Analysis
4.4.2. DNA Gel Electrophoresis
4.5. Measurement of Caspases Activity
4.6. Cell Cycle Analysis
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yuan, B.; Yoshino, Y.; Kaise, T.; Toyoda, H. Application of Arsenic Trioxide Therapy for Patients with Leukaemia. In Biological Chemistry of Arsenic, Antimony and Bismuth; Sun, H., Ed.; John Wiley Sons, Ltd.: Chichester, UK, 2010; pp. 263–292. [Google Scholar]
- Hu, X.M.; Yuan, B.; Tanaka, S.; Song, M.M.; Onda, K.; Tohyama, K.; Zhou, A.X.; Toyoda, H.; Hirano, T. Arsenic disulfide-triggered apoptosis and erythroid differentiation in myelodysplastic syndrome and acute myeloid leukemia cell lines. Hematology 2014, 19, 352–360. [Google Scholar] [CrossRef]
- Hu, X.M.; Yuan, B.; Tanaka, S.; Zhou, Q.; Onda, K.; Toyoda, H.; Hirano, T. Involvement of oxidative stress associated with glutathione depletion and p38 mitogen-activated protein kinase activation in arsenic disulfide-induced differentiation in HL-60 cells. Leuk Lymphoma 2014, 55, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Onda, K.; Sugiyama, K.; Yuan, B.; Tanaka, S.; Takagi, N.; Hirano, T. Antitumor effects of arsenic disulfide on the viability, migratory ability, apoptosis and autophagy of breast cancer cells. Oncol. Rep. 2019, 41, 27–42. [Google Scholar]
- Zhao, Y.; Onda, K.; Yuan, B.; Tanaka, S.; Kiyomi, A.; Sugiyama, K.; Sugiura, M.; Takagi, N.; Hirano, T. Arsenic disulfideinduced apoptosis and its potential mechanism in two and threedimensionally cultured human breast cancer MCF7 cells. Int. J. Oncol. 2018, 52, 1959–1971. [Google Scholar] [PubMed]
- Iriyama, N.; Yoshino, Y.; Yuan, B.; Horikoshi, A.; Hirabayashi, Y.; Hatta, Y.; Toyoda, H.; Takeuchi, J. Speciation of arsenic trioxide metabolites in peripheral blood and bone marrow from an acute promyelocytic leukemia patient. J. Hematol. Oncol. 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, Y.; Yuan, B.; Miyashita, S.I.; Iriyama, N.; Horikoshi, A.; Shikino, O.; Toyoda, H.; Kaise, T. Speciation of arsenic trioxide metabolites in blood cells and plasma of a patient with acute promyelocytic leukemia. Anal. Bioanal. Chem. 2009, 393, 689–697. [Google Scholar] [CrossRef]
- Kiguchi, T.; Yoshino, Y.; Yuan, B.; Yoshizawa, S.; Kitahara, T.; Akahane, D.; Gotoh, M.; Kaise, T.; Toyoda, H.; Ohyashiki, K. Speciation of arsenic trioxide penetrates into cerebrospinal fluid in patients with acute promyelocytic leukemia. Leuk. Res. 2010, 34, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Aposhian, H.V.; Zakharyan, R.A.; Avram, M.D.; Sampayo-Reyes, A.; Wollenberg, M.L. A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species. Toxicol. Appl. Pharmacol. 2004, 198, 327–335. [Google Scholar]
- Kumagai, Y.; Sumi, D. Arsenic: Signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 243–262. [Google Scholar] [CrossRef]
- Bansal, N.; Farley, N.J.; Wu, L.; Lewis, J.; Youssoufian, H.; Bertino, J.R. Darinaparsin inhibits prostate tumor-initiating cells and Du145 xenografts and is an inhibitor of hedgehog signaling. Mol. Cancer Ther. 2015, 14, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Garnier, N.; Petruccelli, L.A.; Molina, M.F.; Kourelis, M.; Kwan, S.; Diaz, Z.; Schipper, H.M.; Gupta, A.; del Rincon, S.V.; Mann, K.K.; et al. The novel arsenical Darinaparsin circumvents BRG1-dependent, HO-1-mediated cytoprotection in leukemic cells. Leukemia 2013, 27, 2220–2228. [Google Scholar] [CrossRef]
- Matulis, S.M.; Morales, A.A.; Yehiayan, L.; Croutch, C.; Gutman, D.; Cai, Y.; Lee, K.P.; Boise, L.H. Darinaparsin induces a unique cellular response and is active in an arsenic trioxide-resistant myeloma cell line. Mol. Cancer Ther. 2009, 8, 1197–1206. [Google Scholar] [CrossRef]
- Ogura, M.; Kim, W.S.; Uchida, T.; Uike, N.; Suehiro, Y.; Ishizawa, K.; Nagai, H.; Nagahama, F.; Sonehara, Y.; Tobinai, K. Phase I studies of darinaparsin in patients with relapsed or refractory peripheral T-cell lymphoma: A pooled analysis of two phase I studies conducted in Japan and Korea. Jpn. J. Clin. Oncol. 2021, 51, 218–227. [Google Scholar] [CrossRef]
- Fukuhara, N.; Kim, W.S.; Yoon, D.H.; Negoro, E.; Yamamoto, K.; Uchida, T.; Izutsu, K.; Terui, Y.; Nakajima, H.; Ando, K.; et al. Asian Multinational Phase II Study of Darinaparsin in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma. Blood 2021, 138 (Suppl. 1), 1376. [Google Scholar] [CrossRef]
- Business Wire. Solasia Pharma: DARVIAS® Injection 135mg (Generic Name: DARINAPARSIN/Development Code: SP-02) Approved in Japan. Available online: https://www.businesswire.com/news/home/20220620005030/en/Solasia-Pharma-DARVIAS®-Injection-135mg-Generic-Name-DARINAPARSIN-Development-Code-SP-02-Approved-in-Japan (accessed on 19 June 2022).
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta 2011, 1807, 735–745. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Yoshino, Y.; Yuan, B.; Okusumi, S.; Aoyama, R.; Murota, R.; Kikuchi, H.; Takagi, N.; Toyoda, H. Enhanced cytotoxic effects of arsenite in combination with anthocyanidin compound, delphinidin, against a human leukemia cell line, HL-60. Chem. Biol. Interact. 2018, 294, 9–17. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Lin, H.; Huang, M.J.; Chow, J.M.; Lin, S.; Liu, H.E. Downregulation of c-Myc is critical for valproic acid-induced growth arrest and myeloid differentiation of acute myeloid leukemia. Leuk. Res. 2007, 31, 1403–1411. [Google Scholar] [CrossRef]
- Mao, A.; Zhao, Q.; Zhou, X.; Sun, C.; Si, J.; Zhou, R.; Gan, L.; Zhang, H. MicroRNA-449a enhances radiosensitivity by downregulation of c-Myc in prostate cancer cells. Sci. Rep. 2016, 6, 27346. [Google Scholar] [CrossRef] [Green Version]
- Pellegata, N.S.; Antoniono, R.J.; Redpath, J.L.; Stanbridge, E.J. DNA damage and p53-mediated cell cycle arrest: A reevaluation. Proc. Natl. Acad. Sci. USA 1996, 93, 15209–15214. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Guo, Z.; Hopkins, C.D.; Wei, N.; Chu, E.; Wipf, P.; Schmitz, J.C. Bis-cyclopropane analog of disorazole C1 is a microtubule-destabilizing agent active in ABCB1-overexpressing human colon cancer cells. Oncotarget 2015, 6, 40866–40879. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.E.; Derbes, R.S.; Ade, C.M.; Ortego, J.C.; Stark, J.; Deininger, P.L.; Roy-Engel, A.M. Heavy Metal Exposure Influences Double Strand Break DNA Repair Outcomes. PLoS ONE 2016, 11, e0151367. [Google Scholar] [CrossRef] [PubMed]
- Reuvers, T.G.A.; Kanaar, R.; Nonnekens, J. DNA Damage-Inducing Anticancer Therapies: From Global to Precision Damage. Cancers 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Ravi, D.; Bhalla, S.; Gartenhaus, R.B.; Crombie, J.; Kandela, I.; Sharma, J.; Mazar, A.; Evens, A.M. The novel organic arsenical darinaparsin induces MAPK-mediated and SHP1-dependent cell death in T-cell lymphoma and Hodgkin lymphoma cells and human xenograft models. Clin. Cancer Res. 2014, 20, 6023–6033. [Google Scholar] [CrossRef] [Green Version]
- Diaz, Z.; Mann, K.K.; Marcoux, S.; Kourelis, M.; Colombo, M.; Komarnitsky, P.B.; Miller, W.H., Jr. A novel arsenical has antitumor activity toward As2O3-resistant and MRP1/ABCC1-overexpressing cell lines. Leukemia 2008, 22, 1853–1863. [Google Scholar] [CrossRef] [Green Version]
- Xiu, M.X.; Liu, Y.M.; Kuang, B.H. The oncogenic role of Jagged1/Notch signaling in cancer. Biomed. Pharmacother. 2020, 129, 110416. [Google Scholar] [CrossRef]
- Aster, J.C.; Blacklow, S.C.; Pear, W.S. Notch signalling in T-cell lymphoblastic leukaemia/lymphoma and other haematological malignancies. J. Pathol. 2011, 223, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.; Alonso-Alonso, R.; Tomás-Roca, L.; Rodríguez-Pinilla, S.M.; Manso-Alonso, R.; Cereceda, L.; Borregón, J.; Villaescusa, T.; Córdoba, R.; Sánchez-Beato, M.; et al. Peripheral T-cell lymphoma: Molecular profiling recognizes subclasses and identifies prognostic markers. Blood Adv. 2021, 5, 5588–5598. [Google Scholar] [CrossRef]
- Xia, J.; Li, Y.; Yang, Q.; Mei, C.; Chen, Z.; Bao, B.; Ahmad, A.; Miele, L.; Sarkar, F.H.; Wang, Z. Arsenic Trioxide Inhibits Cell Growth and Induces Apoptosis through Inactivation of Notch Signaling Pathway in Breast Cancer. Int. J. Mol. Sci. 2012, 13, 9627–9641. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhao, S.; Li, Q.; Li, Y.; Kawamoto, K. Arsenic trioxide-mediated Notch pathway inhibition depletes the cancer stem-like cell population in gliomas. Cancer Lett. 2010, 292, 64–72. [Google Scholar] [CrossRef]
- Mann, K.K.; Wallner, B.; Lossos, I.S.; Miller, W.H., Jr. Darinaparsin: A novel organic arsenical with promising anticancer activity. Expert Opin. Investig. Drugs 2009, 18, 1727–1734. [Google Scholar] [CrossRef]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Q.; Wang, X.; Xu, Y.; Xu, R.; Han, M.; Huang, B.; Chen, A.; Qiu, C.; Sun, T.; et al. Bufalin enhances radiosensitivity of glioblastoma by suppressing mitochondrial function and DNA damage repair. Biomed. Pharmacother. 2017, 94, 627–635. [Google Scholar] [CrossRef]
- Xin, X.; Wen, T.; Gong, L.B.; Deng, M.M.; Hou, K.Z.; Xu, L.; Shi, S.; Qu, X.J.; Liu, Y.P.; Che, X.F.; et al. Inhibition of FEN1 Increases Arsenic Trioxide-Induced ROS Accumulation and Cell Death: Novel Therapeutic Potential for Triple Negative Breast Cancer. Front. Oncol. 2020, 10, 425. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Luo, L.; Zhu, H.; Yang, H.; Zhang, Y.; Wu, H.; Sun, H.; Jiang, F.; Kathera, C.S.; Liu, L.; et al. FEN1 promotes tumor progression and confers cisplatin resistance in non-small-cell lung cancer. Mol. Oncol. 2017, 11, 640–654. [Google Scholar] [CrossRef] [Green Version]
- Heldt, F.S.; Barr, A.R.; Cooper, S.; Bakal, C.; Novak, B. A comprehensive model for the proliferation-quiescence decision in response to endogenous DNA damage in human cells. Proc. Natl. Acad. Sci. USA 2018, 115, 2532–2537. [Google Scholar] [CrossRef] [Green Version]
- Mandigo, A.C.; Yuan, W.; Xu, K.; Gallagher, P.; Pang, A.; Guan, Y.F.; Shafi, A.A.; Thangavel, C.; Sheehan, B.; Bogdan, D.; et al. RB/E2F1 as a Master Regulator of Cancer Cell Metabolism in Advanced Disease. Cancer Discov. 2021, 11, 2334–2353. [Google Scholar] [CrossRef]
- Mao, A.; Liu, Y.; Wang, Y.; Zhao, Q.; Zhou, X.; Sun, C.; Di, C.; Si, J.; Gan, L.; Zhang, H. miR-449a enhances radiosensitivity through modulating pRb/E2F1 in prostate cancer cells. Tumour. Biol. 2016, 37, 4831–4840. [Google Scholar] [CrossRef]
- Hosein, P.J.; Craig, M.D.; Tallman, M.S.; Boccia, R.V.; Hamilton, B.L.; Lewis, J.J.; Lossos, I.S. A multicenter phase II study of darinaparsin in relapsed or refractory Hodgkin’s and non-Hodgkin’s lymphoma. Am. J. Hematol. 2012, 87, 111–114. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Johnson, N.; Garnier, N.; Kwan, S.; Yao, L.; Cocolakis, E.; Hebert, J.; Morgan, R.A.; Paquet, E.; Callahan, K.P.; et al. Monitoring Response and Resistance to the Novel Arsenical Darinaparsin in an AML Patient. Front. Pharmacol. 2013, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lin, Z.; Zhao, N.; Zhou, L.; Liu, F.; Cichacz, Z.; Zhang, L.; Zhan, Q.; Zhao, X. Receptor interactive protein kinase 3 promotes Cisplatin-triggered necrosis in apoptosis-resistant esophageal squamous cell carcinoma cells. PLoS ONE 2014, 9, e100127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Doi, H.; Saar, M.; Santos, J.; Li, X.; Peehl, D.M.; Knox, S.J. Radioprotection and cell cycle arrest of intestinal epithelial cells by darinaparsin, a tumor radiosensitizer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zheng, M.; Lu, R.; Du, J.; Zhao, Q.; Li, Z.; Li, Y.; Zhang, S. The role of CDC25C in cell cycle regulation and clinical cancer therapy: A systematic review. Cancer Cell Int. 2020, 20, 213. [Google Scholar] [CrossRef] [PubMed]
- Thanasoula, M.; Escandell, J.M.; Suwaki, N.; Tarsounas, M. ATM/ATR checkpoint activation downregulates CDC25C to prevent mitotic entry with uncapped telomeres. Embo J. 2012, 31, 3398–3410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Zhao, W.; Huang, Z.N.; Yang, S.M.; Wang, L.J.; Jiang, Y.; Zhou, Z.S.; Zheng, M.Y.; Jiang, J.L.; Li, S.H.; et al. Evaluation of Novel N-(piperidine-4-yl)benzamide Derivatives as Potential Cell Cycle Inhibitors in HepG2 Cells. Chem. Biol. Drug Des. 2015, 86, 223–231. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, R.C.; Verfaillie, C.M. Abnormal integrin-mediated regulation of chronic myelogenous leukemia CD34+ cell proliferation: BCR/ABL up-regulates the cyclin-dependent kinase inhibitor, p27Kip, which is relocated to the cell cytoplasm and incapable of regulating cdk2 activity. Proc. Natl. Acad. Sci. USA 2000, 97, 10538–10543. [Google Scholar] [CrossRef] [Green Version]
- Wegiel, B.; Ekberg, J.; Talasila, K.M.; Jalili, S.; Persson, J.L. The role of VEGF and a functional link between VEGF and p27Kip1 in acute myeloid leukemia. Leukemia 2009, 23, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yuan, B.; Bian, B.; Zhao, H.; Kiyomi, A.; Hayashi, H.; Iwatani, Y.; Sugiura, M.; Takagi, N. Cytotoxic Effects of Hellebrigenin and Arenobufagin Against Human Breast Cancer Cells. Front. Oncol. 2021, 11, 711220. [Google Scholar] [CrossRef]
- Yuan, B.; Okusumi, S.; Yoshino, Y.; Moriyama, C.; Tanaka, S.; Hirano, T.; Takagi, N.; Toyoda, H. Delphinidin induces cytotoxicity and potentiates cytocidal effect in combination with arsenite in an acute promyelocytic leukemia NB4 cell line. Oncol. Rep. 2015, 34, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, H.; Yuan, B.; Yuhara, E.; Takagi, N.; Toyoda, H. Involvement of histone H3 phosphorylation through p38 MAPK pathway activation in casticin-induced cytocidal effects against the human promyelocytic cell line HL-60. Int. J. Oncol. 2013, 43, 2046–2056. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Yao, M.; Wang, X.; Sato, A.; Okazaki, A.; Komuro, H.; Hayashi, H.; Toyoda, H.; Pei, X.; Hu, X.; et al. Antitumor activity of arsenite in combination with tetrandrine against human breast cancer cell line MDA-MB-231 in vitro and in vivo. Cancer Cell Int. 2018, 18, 113. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Ohyama, K.; Takeichi, M.; Toyoda, H. Direct contribution of inducible nitric oxide synthase expression to apoptosis induction in primary smooth chorion trophoblast cells of human fetal membrane tissues. Int. J. Biochem. Cell Biol. 2009, 41, 1062–1069. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Kikuchi, H.; Li, J.; Kawabata, A.; Yao, K.; Takagi, N.; Okazaki, M. Cytotoxic Effects of Darinaparsin, a Novel Organic Arsenical, against Human Leukemia Cells. Int. J. Mol. Sci. 2023, 24, 2282. https://doi.org/10.3390/ijms24032282
Yuan B, Kikuchi H, Li J, Kawabata A, Yao K, Takagi N, Okazaki M. Cytotoxic Effects of Darinaparsin, a Novel Organic Arsenical, against Human Leukemia Cells. International Journal of Molecular Sciences. 2023; 24(3):2282. https://doi.org/10.3390/ijms24032282
Chicago/Turabian StyleYuan, Bo, Hidetomo Kikuchi, Jingmei Li, Atsushi Kawabata, Kozo Yao, Norio Takagi, and Mari Okazaki. 2023. "Cytotoxic Effects of Darinaparsin, a Novel Organic Arsenical, against Human Leukemia Cells" International Journal of Molecular Sciences 24, no. 3: 2282. https://doi.org/10.3390/ijms24032282