Physicochemical Properties of Novel Copolymers of Quaternary Ammonium UDMA Analogues, Bis-GMA, and TEGDMA
Abstract
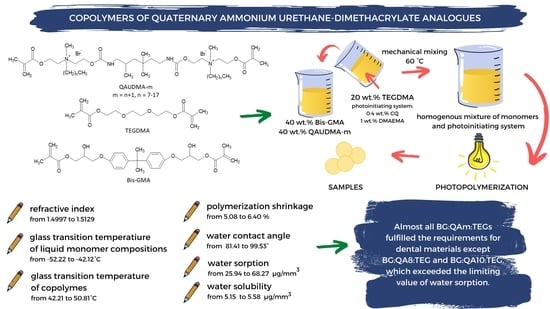
:1. Introduction
2. Results
2.1. Properties of Liquid Monomer Compositions
2.2. Properties of Copolymers
3. Discussion
3.1. Properties of Liquid Monomer Compositions
3.2. Properties of Copolymers
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation
4.3. Refractive Index
4.4. Density and Polymerization Shrinkage
4.5. Glass Transition Temperature
4.6. Water Contact Angle
4.7. Water Sorption and Solubility
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial Quaternary Ammonium Compounds in Dental Materials: A Systematic Review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, S.; Zhou, X.; Wang, H.; Xu, H.H.K.; Cheng, L. The Use of Quaternary Ammonium to Combat Dental Caries. Materials 2015, 8, 3532–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwaśniewska, D.; Chen, Y.L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, F.; Sun, X.; Dong, Y.; Lin, P.T.; Yu, H.H.; Xiao, Y.H.; Chai, Z.G.; Xing, X.D.; Chen, J.H. Antibacterial Activity of a Modified Unfilled Resin Containing a Novel Polymerizable Quaternary Ammonium Salt MAE-HB. Sci. Rep. 2016, 6, 33858. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, Y.H.; Xing, X.D.; Li, F.; Ma, S.; Qi, L.L.; Chen, J.H. Antibacterial Activity and Cytotoxicity of Two Novel Cross-Linking Antibacterial Monomers on Oral Pathogens. Arch. Oral Biol. 2011, 56, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial Activity and Bonding Characteristics of an Adhesive Resin Containing Antibacterial Monomer MDPB. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Li, F.; Li, F.; Wu, D.; Ma, S.; Gao, J.; Li, Y.; Xiao, Y.; Chen, J. The Effect of an Antibacterial Monomer on the Antibacterial Activity and Mechanical Properties of a Pit-and-Fissure Sealant. J. Am. Dent. Assoc. 2011, 142, 184–193. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Chen, J.H.; Fang, M.; Xing, X.D.; Wang, H.; Wang, Y.J.; Li, F. Antibacterial Effects of Three Experimental Quaternary Ammonium Salt (QAS) Monomers on Bacteria Associated with Oral Infections. J. Oral Sci. 2008, 50, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Chai, Z.; Li, F.; Fang, M.; Wang, Y.; Ma, S.; Xiao, Y.; Huang, L.; Chen, J. The Bonding Property and Cytotoxicity of a Dental Adhesive Incorporating a New Antibacterial Monomer. J. Oral Rehabil. 2011, 38, 849–856. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and Characterization of Dimethacrylates Containing Quaternary Ammonium Functionalities for Dental Applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of Quaternary Ammonium Chain Length on Antibacterial Bonding Agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Wu, D.; Fu, R. Studies on the Synthesis and Antibacterial Activities of Polymeric Quaternary Ammonium Salts from Dimethylaminoethyl Methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Manouchehri, F.; Sadeghi, B.; Najafi, F.; Mosslemin, M.H.; Niakan, M. Synthesis and Characterization of Novel Polymerizable Bis-Quaternary Ammonium Dimethacrylate Monomers with Antibacterial Activity as an Efficient Adhesive System for Dental Restoration. Polym. Bull. 2019, 76, 1295–1315. [Google Scholar] [CrossRef]
- Yanwei, Y.; Li, H.; Yan, D.; Hongchen, Z.; Wei, Z.; Jinghao, B.; Jingjing, W.; Yan, L.; Jing, G.; Jihua, C. In Vitro Antibacterial Activity of a Novel Resin-Based Pulp Capping Material Containing the Quaternary Ammonium Salt Mae-Db and Portland Cement. PLoS ONE 2014, 9, e0112549. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Comparison of Quaternary Ammonium-Containing with Nano-Silver-Containing Adhesive in Antibacterial Properties and Cytotoxicity. Dent. Mater. 2013, 29, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Cherchali, F.Z.; Mouzali, M.; Tommasino, J.B.; Decoret, D.; Attik, N.; Aboulleil, H.; Seux, D.; Grosgogeat, B. Effectiveness of the DHMAI Monomer in the Development of an Antibacterial Dental Composite. Dent. Mater. 2017, 33, 1381–1391. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of Methacrylate Monomers with Antibacterial Effects against S. Mutans. Molecules 2011, 16, 9755–9763. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, P.; Ghaemy, M.; Mohseni, M. Synthesis and Characterization of Photo-Curable Bis-Quaternary Ammonium Dimethacrylate with Antimicrobial Activity for Dental Restoration Materials. Eur. Polym. J. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Liang, X.; Söderling, E.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Optimizing the Concentration of Quaternary Ammonium Dimethacrylate Monomer in Bis-GMA/TEGDMA Dental Resin System for Antibacterial Activity and Mechanical Properties. J. Mater. Sci. Mater. Med. 2014, 25, 1387–1393. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Q.; Liu, F.; He, J.; Lin, Z. Synthesis of Novel Antibacterial Monomers (UDMQA) and Their Potential Application in Dental Resin. J. Appl. Polym. Sci. 2013, 129, 3373–3381. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, Z.; Liang, X.; Liu, F.; He, J. Preparation and Characterization of Antibacterial Dental Resin with UDMQA-12. Adv. Polym. Technol. 2014, 33, 21395. [Google Scholar] [CrossRef]
- Huang, Q.T.; He, J.W.; Lin, Z.M.; Liu, F.; Lassila, L.V.J.; Vallittu, P.K. Physical and Chemical Properties of an Antimicrobial Bis-GMA Free Dental Resin with Quaternary Ammonium Dimethacrylate Monomer. J. Mech. Behav. Biomed. Mater. 2016, 56, 68–76. [Google Scholar] [CrossRef]
- Chrószcz, M.W.; Barszczewska-Rybarek, I.M. Synthesis and Characterization of Novel Quaternary Ammonium Urethane-Dimethacrylate Monomers—A Pilot Study. Int. J. Mol. Sci. 2021, 22, 8842. [Google Scholar] [CrossRef] [PubMed]
- Chrószcz, M.W.; Barszczewska-Rybarek, I.M.; Kazek-K, A. Novel Antibacterial Copolymers Based on Quaternary Ammonium Urethane-Dimethacrylate Analogues and Triethylene Glycol Dimethacrylate. Int. J. Mol. Sci. 2022, 23, 4954. [Google Scholar] [CrossRef] [PubMed]
- Chrószcz-Porębska, M.W.; Barszczewska-Rybarek, I.M.; Chladek, G. Characterization of the Mechanical Properties, Water Sorption, and Solubility of Antibacterial Copolymers of Quaternary Ammonium Urethane-Dimethacrylates and Triethylene Glycol Dimethacrylate. Materials 2022, 15, 5530. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Silikas, N.; Satterthwaite, J.D. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent. Mater. 2013, 29, e213–e217. [Google Scholar] [CrossRef]
- Ensaff, H.; O’Doherty, D.M.; Jacobsen, P.H. Polymerization Shrinkage of Dental Composite Resins. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2001, 215, 367–375. [Google Scholar] [CrossRef]
- Moraes, J.C.S.; Sostena, M.M.D.S.; Grandini, C.R. The Glass Transition Temperature in Dental Composites. In Metal, Ceramic and Polymeric Composites for Various Uses; Cuppoletti, J., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- ISO 4049:2019; Dentistry—Polymer Based Restorative Materials. International Standard Organisation: London, UK, 2019.
- Bociong, K.; Szczesio, A.; Sokolowski, K.; Domarecka, M.; Sokolowski, J.; Krasowski, M.; Lukomska-Szymanska, M. The Influence of Water Sorption of Dental Light-Cured Composites on Shrinkage Stress. Materials 2017, 10, 1142. [Google Scholar] [CrossRef]
- Kumar, N.; Sangi, L. Water Sorption, Solubility, and Resultant Change in Strength among Three Resin-Based Dental Composites. J. Investig. Clin. Dent. 2014, 5, 144–150. [Google Scholar] [CrossRef]
- Chrószcz-Porębska, M.W.; Barszczewska-Rybarek, I.M.; Kazek-Kęsik, A.; Chladek, G. Novel mechanically strong and antibacterial dimethacrylate copolymers based on quaternary ammonium urethane-dimethacrylate analogues. Dent. Mater. 2022; submitted. [Google Scholar]
- Meng, Z.; Yao, X.S.; Yao, H.; Liang, Y.; Liu, T.; Li, Y.; Wang, G.; Lan, S. Measurement of the Refractive Index of Human Teeth by Optical Coherence Tomography. J. Biomed. Opt. 2009, 14, 034010. [Google Scholar] [CrossRef]
- Manappallil, J.J. Basic Dental Materials, 4th ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2015. [Google Scholar]
- Véchambre, C.; Buléon, A.; Chaunier, L.; Gauthier, C.; Lourdin, D. Understanding the Mechanisms Involved in Shape Memory Starch: Macromolecular Orientation, Stress Recovery and Molecular Mobility. Macromolecules 2011, 44, 9384–9389. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. Structure–Property Relationships in Dimethacrylate Networks Based on Bis-GMA, UDMA and TEGDMA. Dent. Mater. 2009, 25, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Schricker, S.R. Composite Resin Polymerization and Relevant Parameters. In Orthodontic Applications of Biomaterials; Woodhead Publishing: Sawston, UK, 2017; pp. 153–170. [Google Scholar] [CrossRef]
- Soares, C.J.; Faria-E-Silva, A.L.; de Paula Rodrigues, M.; Fernandes Vilela, A.B.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization Shrinkage Stress of Composite Resins and Resin Cements—What Do We Need to Know? Braz. Oral Res. 2017, 31, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, D.C. Adhesives and Sealants. In Biomaterials Science: An Introduction to Materials, 3rd ed.; Ratner, B., Hoffman, A., Schoen, F., Lemons, J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 889–904. [Google Scholar]
- Wang, R.-M.; Zheng, S.-R.; Zheng, Y.-P. Matrix Materials. In Polymer Matrix Composites and Technology; Woodhead Publishing: Sawston, UK, 2011; pp. 101–548. [Google Scholar] [CrossRef]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. Clinical Methods: The History, Hysical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin Based Restorative Dental Materials: Characteristics and Future Perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Farooq, I.; Ali, S.; Al-Saleh, S.; Alhamdan, E.M.; Alrefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives—A Review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef] [PubMed]
- Ayar, M.K. A Review of Ethanol Wet-Bonding: Principles and Techniques. Eur. J. Dent. 2016, 10, 155–159. [Google Scholar] [CrossRef]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Photoresponsive Membranes. Interface Sci. Technol. 2018, 25, 115–144. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, L.; Guan, S.; Gao, G.; Cheng, Y.; Ren, X.; Wang, Y. The Effect of Hydrophobic Alkyl Chain Length on the Mechanical Properties of Latex Particle Hydrogels. RSC Adv. 2017, 7, 44673–44679. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, I.; Pakiet, M.; Szulc, A.; Koziróg, A. Antimicrobial Activity of Gemini Surfactants with Azapolymethylene Spacer. Molecules 2020, 25, 4054. [Google Scholar] [CrossRef]
- Feilzer, A.J.; Kakaboura, A.I.; de Gee, A.J.; Davidson, C.L. The Influence of Water Sorption on the Development of Setting Shrinkage Stress in Traditional and Resin-Modified Glass Ionomer Cements. Dent. Mater. 1995, 11, 186–190. [Google Scholar] [CrossRef]
- Al-Bader, R.M.; Ziadan, K.M.; Al-Ajely, M.S. Water Adsorption Characteristics of New Dental Composites. Int. J. Med. Res. Health Sci. 2015, 4, 281. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Beyth, N.; Weiss, E.I.; Domb, A.J. Antibacterial Effect of Composite Resins Containing Quaternary Ammonium Polyethyleneimine Nanoparticles. J. Nanoparticle Res. 2010, 12, 591–603. [Google Scholar] [CrossRef]
- ISO 489:1999; Plastics—Determination of Refractive Index. International Standard Organisation: London, UK, 1999.
- ISO 1675:2002; Plastics—Liquid Resins—Determination of Density by the Pyknometer Method. International Standard Organisation: London, UK, 2002.
- ISO 11357-2:2020; Plastics—Differential Scanning Calorimetry (DSC)—Part 2: Determination of Glass Transition Temperature and Step Height. International Standard Organisation: London, UK, 2020.





| Sample Name | Cm | MW (g/mol) | xDB (kg/mol) | RI1 | dm (g/cm3) | |
|---|---|---|---|---|---|---|
| Average | SD | |||||
| BG:QA8:TEG | C8 | 528.74 | 3.78 | 1.4997 | 1.154 | 0.005 |
| BG:QA10:TEG | C10 | 535.11 | 3.74 | 1.4982 | 1.142 | 0.006 |
| BG:QA12:TEG | C12 | 540.95 | 3.70 | 1.5128 a,b | 1.127 | 0.005 |
| BG:QA14:TEG | C14 | 546.33 | 3.66 | 1.5129 a,c | 1.116 a–d | 0.007 |
| BG:QA16:TEG | C16 | 551.29 | 3.63 | 1.5099 | 1.113 a,e–g | 0.010 |
| BG:QA18:TEG | C18 | 555.89 | 3.60 | 1.5097 | 1.099 b,e,h,i | 0.013 |
| BG:UD:TEG | - | 389.27 | 5.14 | 1.5127 b,c | 1.115 c,f,h | 0.007 |
| BG:TEG | - | 429.39 | 4.66 | 1.5048 | 1.102 d,g,i | 0.005 |
| Sample Name | dp (g/cm3) | Se (%) | St (%) | DC [32] | ||
|---|---|---|---|---|---|---|
| Average | SD | Average | SD | |||
| BG:QA8:TEG | 1.216 a | 0.007 | 5.08 | 0.40 | 9.81 | 0.59 |
| BG:QA10:TEG | 1.208 b | 0.004 | 5.48 a | 0.37 | 9.60 | 0.60 |
| BG:QA12:TEG | 1.200 c | 0.002 | 6.07 a–d | 0.49 | 9.38 | 0.61 |
| BG:QA14:TEG | 1.189 d | 0.002 | 6.14 b,e,f | 0.41 | 9.18 | 0.63 |
| BG:QA16:TEG | 1.186 d | 0.005 | 6.24 c,e,g | 0.54 | 9.04 | 0.66 |
| BG:QA18:TEG | 1.174 | 0.004 | 6.40 d,f,g | 0.48 | 8.90 | 0.68 |
| BG:UD:TEG | 1.215 a | 0.002 | 8.35 h | 0.23 | 12.90 | 0.64 |
| BG:TEG | 1.206 b,c | 0.003 | 8.07 h | 0.80 | 11.61 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrószcz-Porębska, M.W.; Barszczewska-Rybarek, I.M.; Chladek, G. Physicochemical Properties of Novel Copolymers of Quaternary Ammonium UDMA Analogues, Bis-GMA, and TEGDMA. Int. J. Mol. Sci. 2023, 24, 1400. https://doi.org/10.3390/ijms24021400
Chrószcz-Porębska MW, Barszczewska-Rybarek IM, Chladek G. Physicochemical Properties of Novel Copolymers of Quaternary Ammonium UDMA Analogues, Bis-GMA, and TEGDMA. International Journal of Molecular Sciences. 2023; 24(2):1400. https://doi.org/10.3390/ijms24021400
Chicago/Turabian StyleChrószcz-Porębska, Marta W., Izabela M. Barszczewska-Rybarek, and Grzegorz Chladek. 2023. "Physicochemical Properties of Novel Copolymers of Quaternary Ammonium UDMA Analogues, Bis-GMA, and TEGDMA" International Journal of Molecular Sciences 24, no. 2: 1400. https://doi.org/10.3390/ijms24021400