The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review
Abstract
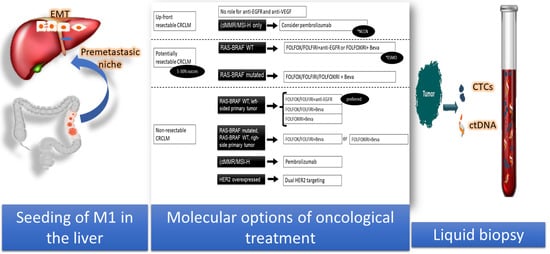
:1. Introduction
2. Metastatic Niche
3. Impact of the Metastasis Molecular Pattern in the Choice of Chemotherapy
3.1. Current Indications for MCA
3.1.1. RAS Wild-Type Cancer
3.1.2. Anti-VEGF Agents
3.1.3. BRAF V600 Mutation
3.1.4. Different BRAF Mutations
3.1.5. Mismatch Repair
3.1.6. HER-2 Amplification
3.2. Initially Unresectable Metastatic Disease
3.3. Resectable Metastases
3.4. Unresectable Metastases
4. The Impact of the Molecular Profile on Follow-Up
4.1. Surveillance for Metastatic Liver Disease
4.2. Circulating Biomarkers
- (a)
- Proteins
- (b)
- Nucleic Acids
- (c)
- Exosomes and microvesicles
- (d)
- Circulating cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEA | carcinoembryonic antigen |
| cfDNA | circulating free DNA |
| CRC | colorectal cancer |
| CRCLM | colorectal cancer liver metastasis |
| CTCs | circulating tumour cells |
| ctDNA | circulating tumour DNA |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| EV | extracellular vesicles |
| MCA | monoclonal antibodies |
| miRNA | micro RNA |
| OS | overall survival |
| PFS | progression free survival |
| TEP | tumour-educated platelet |
| VEGF | vascular endothelial growth factor |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Borner, M.M. Neoadjuvant chemotherapy for unresectable liver metastases of colorectal cancer—Too good to be true? Editorial. Ann. Oncol. 1999, 10, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Recently Updated NCCN Clinical Practice Guidelines in OncologyTM. Available online: https://www.nccn.org/professionals/physician_gls/recently_updated.aspx (accessed on 19 March 2020).
- Nordlinger, B.; Guiget, M.; Vaillant, J.C.; Balladur, P.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318; discussion 318–321. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Dvorchik, I.; Madariaga, J.R.; Wallis Marsh, J.; Dodson, F.; Bonham, A.C.; Geller, D.A.; Gayowski, T.J.; Fung, J.J.; Starzl, T.E. Hepatic resection for metastatic colorectal adenocarcinoma: A proposal of a prognostic scoring system. J. Am. Coll. Surg. 1999, 189, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, E.K.; Vauthey, J.N.; Ellis, L.M.; Ellis, V.; Pollock, R.; Broglio, K.R.; Hess, K.; Curley, S.A.; Dale, P.S.; Howard, R.J.; et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004, 239, 818–827. [Google Scholar] [CrossRef]
- Fernandez, F.G.; Drebin, J.A.; Linehan, D.C.; Dehdashti, F.; Siegel, B.A.; Strasberg, S.M.; Fong, Y.; Wanebo, H.J.; Henderson, J.M.; Pinson, C.W. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann. Surg. 2004, 240, 438–450. [Google Scholar] [CrossRef]
- Muratore, A.; Zorzi, D.; Bouzari, H.; Amisano, M.; Massucco, P.; Sperti, E.; Capussotti, L. Asymptomatic colorectal cancer with un-resectable liver metastases: Immediate colorectal resection or up-front systemic chemotherapy? Ann. Surg. Oncol. 2007, 14, 766–770. [Google Scholar] [CrossRef]
- Alberts, S.R.; Horvath, W.L.; Sternfeld, W.C.; Goldberg, R.M.; Mahoney, M.R.; Dakhil, S.R.; Levitt, R.; Rowland, K.; Nair, S.; Sargent, D.J.; et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J. Clin. Oncol. 2005, 23, 9243–9249. [Google Scholar] [CrossRef] [PubMed]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.O.; Raab, H.R.; Lordick, F.; Hartmann, J.T.; Lang, H.; Frilling, A.; Stoehlmacher, J.; Weitz, J.; et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010, 11, 38–47. [Google Scholar] [CrossRef]
- Weeks, J.C.; Catalano, P.J.; Cronin, A.; Finkelman, M.D.; Mack, J.W.; Keating, N.L.; Schrag, D. Patients’ expectations about effects of chemotherapy for advanced cancer. N. Engl. J. Med. 2012, 367, 1616–1625. [Google Scholar] [CrossRef] [Green Version]
- Gruenberger, T.; Bridgewater, J.; Chau, I.; Garcia Alfonso, P.; Rivoire, M.; Mudan, S.; Lasserre, S.; Hermann, F.; Waterkamp, D.; Adam, R. Bevacizumab Plus mFOLFOX-6 or FOLFOXIRI in Patients with Initially Unresectable Liver Metastases From Colorectal Cancer: The OLIVIA Multinational Randomised Phase II Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 702–708. [Google Scholar] [CrossRef]
- Ye, L.C.; Liu, T.S.; Ren, L.; Wei, Y.; Zhu, D.X.; Zai, S.Y.; Ye, Q.H.; Yu, Y.; Xu, B.; Qin, X.Y.; et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J. Clin. Oncol. 2013, 31, 1931–1938. [Google Scholar] [CrossRef] [Green Version]
- Drew, J.; Machesky, L.M. The liver metastatic niche: Modelling the extracellular matrix in metastasis. Dis. Model. Mech. 2021, 14, dmm048801. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Xiang, D.M.; Sun, W.; Ning, B.F.; Zhou, T.F.; Li, X.F.; Zhong, W.; Cheng, Z.; Xia, M.Y.; Wang, X.; Deng, X.; et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut 2018, 67, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Celià-Terrassa, T.; Kang, Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016, 30, 892–908. [Google Scholar] [CrossRef] [Green Version]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Ali, S.R.; Jordan, M.; Nagarajan, P.; Amit, M. Nerve Density and Neuronal Biomarkers in Cancer. Cancers 2022, 14, 4817. [Google Scholar] [CrossRef]
- Naba, A.; Pearce, O.M.T.; Del Rosario, A.; Ma, D.; Ding, H.; Rajeeve, V.; Cutillas, P.R.; Balkwill, F.R.; Hynes, R.O. Characterization of the Extracellular Matrix of Normal and Diseased Tissues Using Proteomics. J. Proteome Res. 2017, 16, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, P.B.; Colpaert, C.; Salgado, R.; Royers, R.; Hellemans, H.; Van Den Heuvel, E.; Goovaerts, G.; Dirix, L.Y.; Van Marck, E. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J. Pathol. 2001, 195, 336–342. [Google Scholar] [CrossRef]
- Feng, W.; Huang, W.; Chen, J.; Qiao, C.; Liu, D.; Ji, X.; Xie, M.; Zhang, T.; Wang, Y.; Sun, M.; et al. CXCL12-mediated HOXB5 overexpression facilitates Colorectal Cancer metastasis through transactivating CXCR4 and ITGB3. Theranostics 2021, 11, 2612–2633. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Kafatos, G.; Taylor, A.; Gastanaga, V.M.; Oliner, K.S.; Hechmati, G.; Terwey, J.H.; Van Krieken, J.H. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: A pooled analysis of randomised controlled trials. Eur. J. Cancer 2015, 51, 1704–1713. [Google Scholar] [CrossRef]
- Baselga, J. The EGFR as a target for anticancer therapy—Focus on cetuximab. Eur. J. Cancer 2001, 37 (Suppl. S4), 16–22. [Google Scholar] [CrossRef] [PubMed]
- Han, C.B.; Li, F.; Ma, J.T.; Zou, H.W. Concordant KRAS mutations in primary and metastatic colorectal cancer tissue specimens: A meta-analysis and systematic review. Cancer Investig. 2012, 30, 741–747. [Google Scholar] [CrossRef]
- Sorich, M.J.; Wiese, M.D.; Rowland, A.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.I.; Tebbutt, N.C.; Kabbinavar, F.; Giantonio, B.J.; Guan, Z.-Z.; Mitchell, L.; Waterkamp, D.; Tabernero, J. Efficacy and safety of bevacizumab in metastatic colorectal cancer: Pooled analysis from seven randomized controlled trials. Oncologist 2013, 18, 1004–1012. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.; Sanoff, H. Systemic Chemotherapy for Metastatic Colorectal Cancer: General Principles—UpToDate. 2022. Available online: https://www.uptodate.com/contents/systemic-chemotherapy-for-metastatic-colorectal-cancer-general-principles?search=GeneralPrincipleslivermetastasis&source=search_result&selectedTitle=5~150&usage_type=default&display_rank=5 (accessed on 25 September 2022).
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef]
- Cohen, R.; Liu, H.; Fiskum, J.; Adams, R.; Chibaudel, B.; Maughan, T.S.; Van Cutsem, E.; Venook, A.; Douillard, J.Y.; Heinemann, V.; et al. BRAF V600E Mutation in First-Line Metastatic Colorectal Cancer: An Analysis of Individual Patient Data From the ARCAD Database. J. Natl. Cancer Inst. 2021, 113, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Renfro, L.A.; Al-Shamsi, H.O.; Schrock, A.B.; Rankin, A.; Zhang, B.Y.; Kasi, P.M.; Voss, J.S.; Leal, A.D.; Sun, J.; et al. Non-V600 BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J. Clin. Oncol. 2017, 35, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, E.; Yoshino, T.; Yamazaki, K.; Muro, K.; Yamaguchi, K.; Nishina, T.; Yuki, S.; Shitara, K.; Bando, H.; Mimaki, S.; et al. Clinical significance of BRAF non-V600E mutations on the therapeutic effects of anti-EGFR monoclonal antibody treatment in patients with pretreated metastatic colorectal cancer: The Biomarker Research for anti-EGFR monoclonal Antibodies by Comprehensive Cancer genomics (BREAC) study. Br. J. Cancer 2017, 117, 1450–1458. [Google Scholar] [CrossRef]
- Johnson, B.; Loree, J.M.; Jacome, A.A.; Mendis, S.; Syed, M.; Morris II, V.K.; Parseghian, C.M.; Dasari, A.; Pant, S.; Raymond, V.M.; et al. Atypical, Non-V600 BRAF Mutations as a Potential Mechanism of Resistance to EGFR Inhibition in Metastatic Colorectal Cancer. JCO Precis. Oncol. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Yaeger, R.; Kotani, D.; Mondaca, S.; Parikh, A.R.; Bando, H.; Van Seventer, E.E.; Taniguchi, H.; Zhao, H.Y.; Thant, C.N.; De Stanchina, E.; et al. Response to Anti-EGFR Therapy in Patients with BRAF non-V600-Mutant Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 7089–7097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet. Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Karan, C.; Tan, E.; Sarfraz, H.; Knepper, T.C.; Walko, C.M.; Felder, S.; Kim, R.; Sahin, I.H. Human Epidermal Growth Factor Receptor 2-Targeting Approaches for Colorectal Cancer: Clinical Implications of Novel Treatments and Future Therapeutic Avenues. JCO Oncol. Pract. 2022, 18, 545–554. [Google Scholar] [CrossRef]
- Park, D.I.; Kang, M.S.; Oh, S.J.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Han, W.K.; Kim, H.; et al. HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int. J. Colorectal Dis. 2007, 22, 491–497. [Google Scholar] [CrossRef]
- Petrelli, F.; Barni, S. Resectability and outcome with anti-EGFR agents in patients with KRAS wild-type colorectal liver-limited metastases: A meta-analysis. Int. J. Colorectal Dis. 2012, 27, 997–1004. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Hartmann, J.T.; de Braud, F.; Schuch, G.; Zubel, A.; Celik, I.; Schlichting, M.; Koralewski, P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1535–1546. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maughan, T.S.; Adams, R.A.; Smith, C.G.; Meade, A.M.; Seymour, M.T.; Wilson, R.H.; Idziaszczyk, S.; Harris, R.; Fisher, D.; Kenny, S.L.; et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet 2011, 377, 2103–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef]
- Tang, W.; Ren, L.; Liu, T.; Ye, Q.; Wei, Y.; He, G.; Lin, Q.; Wang, X.; Wang, M.; Liang, F.; et al. Bevacizumab Plus mFOLFOX6 Versus mFOLFOX6 Alone as First-Line Treatment for RAS Mutant Unresectable Colorectal Liver-Limited Metastases: The BECOME Randomized Controlled Trial. J. Clin. Oncol. 2020, 38, 3175–3184. [Google Scholar] [CrossRef]
- Modest, D.P.; Martens, U.M.; Riera-Knorrenschild, J.; Greeve, J.; Florschütz, A.; Wessendorf, S.; Ettrich, T.; Kanzler, S.; Nörenberg, D.; Ricke, J.; et al. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: The randomized, open-label, phase II Volfi study (AIO KRK0109). J. Clin. Oncol. 2019, 37, 3401–3411. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [Green Version]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.A.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; Griffiths, G.O.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet. Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef]
- Clark, J.; Sanoff, H. Systemic Therapy for Nonoperable Metastatic Colorectal Cancer: Selecting the Initial Therapeutic Approache. Available online: https://www.uptodate.com/contents/systemic-therapy-for-nonoperable-metastatic-colorectal-cancer-selecting-the-initial-therapeutic-approach?search=unresectablelivermetastasiscolorectalinitial&source=search_result&selectedTitle=6~150&usage_type=default& (accessed on 25 September 2022).
- Hochster, H.S.; Hart, L.L.; Ramanathan, R.K.; Childs, B.H.; Hainsworth, J.D.; Cohn, A.L.; Wong, L.; Fehrenbacher, L.; Abubakr, Y.; Saif, M.W.; et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE Study. J. Clin. Oncol. 2008, 26, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Stathopoulos, G.P.; Batziou, C.; Trafalis, D.; Koutantos, J.; Batzios, S.; Stathopoulos, J.; Legakis, J.; Armakolas, A. Treatment of colorectal cancer with and without bevacizumab: A phase III study. Oncology 2010, 78, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Nanni, O.; Tassinari, D.; Turci, D.; Cavanna, L.; Fontana, A.; Ruscelli, S.; Mucciarini, C.; Lorusso, V.; Ragazzini, A.; et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: Final results for first-line treatment from the ITACa randomized clinical trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Köhne, C.H.; Hofheinz, R.; Mineur, L.; Letocha, H.; Greil, R.; Thaler, J.; Fernebro, E.; Gamelin, E.; DeCosta, L.; Karthaus, M. First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 65–72. [Google Scholar] [CrossRef]
- Berlin, J.; Posey, J.; Tchekmedyian, S.; Hu, E.; Chan, D.; Malik, I.; Yang, L.; Amado, R.G.; Randolph Hecht, J. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin. Colorectal Cancer 2007, 6, 427–432. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Wang, L.; Xu, J.; Cheng, Y.; Bai, Y.; Li, W.; Xu, N.; Lin, L.Z.; Wu, Q.; et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J. Clin. Oncol. 2018, 36, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Tveit, K.M.; Guren, T.; Glimelius, B.; Pfeiffer, P.; Sorbye, H.; Pyrhonen, S.; Sigurdsson, F.; Kure, E.; Ikdahl, T.; Skovlund, E.; et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J. Clin. Oncol. 2012, 30, 1755–1762. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Colorectal Cancer. NICE guideline NG151. 2020. Available online: https://www.nice.org.uk/guidance/ng151 (accessed on 25 September 2022).
- Lee, J.H.; Lee, S.-W. The Roles of Carcinoembryonic Antigen in Liver Metastasis and Therapeutic Approaches. Gastroenterol. Res. Pract. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2021. [Google Scholar] [CrossRef]
- Pericleous, S.; Bhogal, R.H.; Mavroeidis, V.K. The Role of Circulating Biomarkers in the Early Detection of Recurrent Colorectal Cancer Following Resection of Liver Metastases. Front. Biosci. 2022, 27, 189. [Google Scholar] [CrossRef]
- Cai, M.; He, H.; Hong, S.; Weng, J. Synergistic diagnostic value of circulating tumor cells and tumor markers CEA/CA19-9 in colorectal cancer. Scand. J. Gastroenterol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Skovlund, E.; Sorbye, H.; Bolstad, N.; Johannes Nustad, K.; Glimelius, B.; Pfeiffer, P.; Kure, E.H.; Johansen, J.S.; Magne Tveit, K.; et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: A BRAF-mutant subset with high CA 19-9 level and poor outcome. Br. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Kumar, R.; Kumar, U.; Kumari, R. Clinical Significance and Role of TK1, CEA, CA 19-9 and CA 72-4 levels in Diagnosis of Colorectal Cancers. Asian Pac. J. Cancer Prev. 2020, 21, 3133–3136. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Ratajczak, K.; Jakiela, S. Toward early cancer detection: Focus on biosensing systems and biosensors for an anti-apoptotic protein survivin and survivin mRNA. Biosens. Bioelectron. 2019, 137, 58–71. [Google Scholar] [CrossRef]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Hairpin-Hairpin Molecular Beacon Interactions for Detection of Survivin mRNA in Malignant SW480 Cells. ACS Appl. Mater. Interfaces 2018, 10, 17028–17039. [Google Scholar] [CrossRef]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Optical Biosensing System for the Detection of Survivin mRNA in Colorectal Cancer Cells Using a Graphene Oxide Carrier-Bound Oligonucleotide Molecular Beacon. Nanomater 2018, 8, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Nakamura, Y.; Taniguchi, H.; Odegaard, J.I.; Nomura, S.; Kojima, M.; Sugimoto, M.; Konishi, M.; Gotohda, N.; Takahashi, S.; et al. Impact of Preoperative Circulating Tumor DNA Status on Survival Outcomes After Hepatectomy for Resectable Colorectal Liver Metastases. Ann. Surg. Oncol. 2021, 28, 4744–4755. [Google Scholar] [CrossRef]
- Bolhuis, K.; van ’t Erve, I.; Mijnals, C.; Delis-Van Diemen, P.M.; Huiskens, J.; Komurcu, A.; Lopez-Yurda, M.; van den Broek, D.; Swijnenburg, R.J.; Meijer, G.A.; et al. Postoperative circulating tumour DNA is associated with pathologic response and recurrence-free survival after resection of colorectal cancer liver metastases. EBioMedicine 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.I.; Wang, Y.; Cohen, J.I.; Li, L.I.; Hong, W.I.; Christie, M.; Li Wong, H.I.; Kosmider, S.I.; Wong, R.I.; Thomson, B.I.; et al. Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: A prospective cohort study. PLoS Med. 2021, 18, e1003620. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Han, L.; Shi, W.J.; Xie, Y.B.; Zhang, Z.G. Diagnostic value of four serum exosome microRNAs panel for the detection of colorectal cancer. World J. Gastrointest. Oncol. 2021, 13, 970–979. [Google Scholar] [CrossRef]
- Hu, H.Y.; Yu, C.H.; Zhang, H.H.; Zhang, S.Z.; Yu, W.Y.; Yang, Y.; Chen, Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int. J. Biol. Macromol. 2019, 132, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Han, B.; Gao, S.; Wang, X.; Wang, Z.; Wang, F.; Zhang, J.; Xu, D.; Sun, B. Exosome-encapsulated microRNAs as circulating biomarkers for colorectal cancer. Oncotarget 2017, 8, 60149–60158. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.Y.; Gu, R.H.; Yan, B. Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J. Cell. Biochem. 2018, 120, 1457–1463. [Google Scholar] [CrossRef]
- Plantureux, L.; Crescence, L.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Effects of platelets on cancer progression. Thromb. Res. 2018, 164 (Suppl. S1), S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.E.; Zurakowski, D.; Italiano, J.E.; Michel, L.V.; Connors, S.; Oenick, M.; D’Amato, R.J.; Klement, G.L.; Folkman, J. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis 2012, 15, 265–273. [Google Scholar] [CrossRef]
- Qian, W.; Ge, X.X.; Wu, J.; Gong, F.R.; Wu, M.Y.; Xu, M.D.; Lian, L.; Wang, W.J.; Li, W.; Tao, M. Prognostic evaluation of resectable colorectal cancer using platelet-associated indicators. Oncol. Lett. 2019, 18, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Jiang, Q.; Li, D.Z.; Zhou, X.; Yu, D.S.; Zhong, J. TIMP1 mRNA in tumor-educated platelets is diagnostic biomarker for colorectal cancer. Aging 2019, 11, 8998–9012. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Rahbari, N.N.; Büchler, M.W.; Koch, M.; Weitz, J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: A meta-analysis. Ann. Surg. Oncol. 2013, 20, 2156–2165. [Google Scholar] [CrossRef]
- Yu, H.; Ma, L.; Zhu, Y.; Li, W.; Ding, L.; Gao, H. Significant diagnostic value of circulating tumour cells in colorectal cancer. Oncol. Lett. 2020, 20, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalmahomed, Z.S.; Mostert, B.; Onstenk, W.; Kraan, J.; Ayez, N.; Gratama, J.W.; Grünhagen, D.; Verhoef, C.; Sleijfer, S. Prognostic value of circulating tumour cells for early recurrence after resection of colorectal liver metastases. Br. J. Cancer 2015, 112, 556–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, A.A.; McNamara, K.; Al-Sukhni, E.; Diskin, J.; Chan, D.; Ash, C.; Lowes, L.E.; Allan, A.L.; Zogopoulos, G.; Moulton, C.A.; et al. Central, But Not Peripheral, Circulating Tumor Cells are Prognostic in Patients Undergoing Resection of Colorectal Cancer Liver Metastases. Ann. Surg. Oncol. 2016, 23, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Liu, L.R.; Yang, X.Y.; Liu, F.; Zhang, Z.G. Serum CA19-9 as a marker of circulating tumor cells in first reflux blood of colorectal cancer patients. Oncotarget 2017, 8, 67918–67932. [Google Scholar] [CrossRef] [PubMed]

| With Cetuximab | ||||||||||||
| Name | DOI | Treatment | KRAS WT LLD patients | Follow-up | PFS | p | OS | p | Resection R0 | p | Treat response | p |
| OPUS 2011 | 10.1093/annonc/mdq632 | FOLFOX-Cetuxi vs. FOLFOX | 48(25/23) | HR 0.64 | 0.39 | HR 0.93 | 0.85 | 16% vs. 4.3% (RR 3.68) | 0.23 | RR 1.94 | 0.02 | |
| CRYSTAL 2011, Van Cutsem | 10.1200/JCO.2010.33.5091 | FOLFIRI-Cetuxi vs. FOLFIRI | 140 (68/72) | 29.7 Mo | HR 0.56 | 0.04 | HR 0.85 | 0.43 | 13.2% vs. 5.5% (RR 2.38) | 0.13 | RR 1.59 | 0.003 |
| MRC Coin 2011 | 10.1016/S0140-6736(11)60613-2 | CAPOX or FOLFOX-Cetuxi vs. CAPOX or FOLFOX | 178 (87/91) | 21 Mo | HR 0.68 | 0.03 | NR | NR | 15% vs. 13% (RR 1.13) | 0.74 | NR | NR |
| Le-Chi Ye 2013 | 10.1200/JCO.2012.44.8308 | FOLFIRI or FOLFOX- Cetuxi vs. FOLFIRI of FOLFOX | 70/68 | 25 Mo | HR 0.60 | 0.004 | HR 0.54 | 0.013 | 25.7% vs. 7.4% (OR 4.37) | 0.004 | 57.1% vs. 29.4% | 0.001 |
| With Panitumumab | ||||||||||||
| Name | DOI | Treatment | KRAS WT LLD patients | Follow-up | PFS | p | OS | p | Resection R0 | p | Treat response | p |
| Douillard 2010 | 10.1200/JCO.2009.27.4860 | FOLFOX-Pani vs. FOLFOX | 116 (60/56) | 13.2 Mo | HR 0.82 | 0.43 | HR 0.93 | 0.81 | 27.8% vs. 17.5% (RR 1.59) | 0.19 | NR | NR |
| With Bevacizumab | ||||||||||||
| Name | DOI | Treatment | KRAS mutated LLD patients | Follow-up | PFS | p | OS | p | Resection R0 | p | Treat response | p |
| BECOME | 10.1200/JCO.20.174 | FOLFOX+Beva vs. FOLFOX | 241 (121/120) | 37 Mo | HR 0.49 | 0.001 | HR 0.71 | 0.31 | 22.3% vs. 5.8% | 0.01 | 54.5% vs. 36.7% | 0.001 |
| Conversion Chemotherapy without Limited Liver Disease | ||||||||||||
| Name | DOI | Treatment | KRAS WT patients | Follow-up | PFS | p | OS | p | Resection | p | Treat response | p |
| VOLFI | 10.1200/JCO.1901340 | FOLFOXIRI-Pani vs. FOLFOXIRI | 96 (63/33) | 44.2 Mo vs. 63.3 Mo | HR 1.07 | 0.76 | HR 0.67 | 0.12 | 33% vs. 12.1% (OR 3.63) | 0.02 | OR 4.469 | 0.004 |
| Salz 2008 | 10.1200/JCO.2007.14.9930 | CAPOX or FOLOFOX-Beva vs. CAPOX or FOLFOX | 700 vs. 701 (no KRAS specified) | 15.6 Mo | HR 0.83 | 0.0023 | HR 0.89 | 0.769 | 8.4% vs. 6.1% | NR | OR 0.9 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, M.-C.; Ramirez-Maldonado, E.; Pueyo-Périz, E.; Memba, R.; Merino, S.; Geoghegan, J.; Jorba, R. The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 1127. https://doi.org/10.3390/ijms24021127
Pavel M-C, Ramirez-Maldonado E, Pueyo-Périz E, Memba R, Merino S, Geoghegan J, Jorba R. The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review. International Journal of Molecular Sciences. 2023; 24(2):1127. https://doi.org/10.3390/ijms24021127
Chicago/Turabian StylePavel, Mihai-Calin, Elena Ramirez-Maldonado, Eva Pueyo-Périz, Robert Memba, Sandra Merino, Justin Geoghegan, and Rosa Jorba. 2023. "The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review" International Journal of Molecular Sciences 24, no. 2: 1127. https://doi.org/10.3390/ijms24021127