Dehydrocorydaline Accelerates Cell Proliferation and Extracellular Matrix Synthesis of TNFα-Treated Human Chondrocytes by Targeting Cox2 through JAK1-STAT3 Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. DHC Facilitated Cell Proliferation of the TNFα-Treated Human Chondrocytes
2.2. DHC Improved ECM Synthesis and Degradation in the TNFα-Treated Human Chondrocytes
2.3. Detection of Differently Expressed Genes, GO, and KEGG Enrichment Analysis
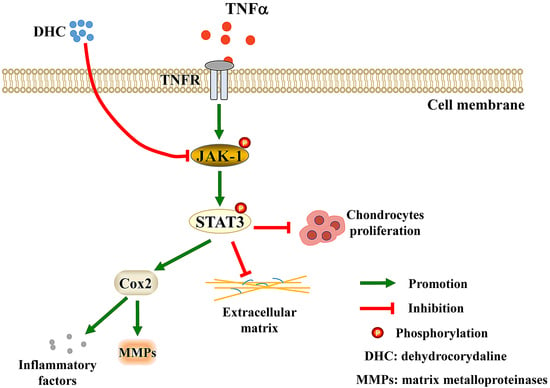
2.4. DHC Inhibited the Phosphorylated Jak1 and Stat3 Expression in the TNFα-Treated Human Chondrocytes
2.5. DHC Ameliorated Cell Proliferation and EXM Synthesis in the TNFα-Treated Human Chondrocytes via JAK1-STAT3 Pathway
2.6. DHC Attenuated Completed ACLT-Induced OA Progression
2.7. DHC Inhibited p-JAK1 and p-STAT3 Expression in the Articular Cartilage In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Experimental Design
4.3. CCK-8 Assay
4.4. EdU Staining and Flow Cytometry
4.5. Quantitative Real-Time Polymerase Chain Reaction
4.6. Western Blotting Assay
4.7. RNA Sequencing
4.8. Immunocytofluorescence Staining
4.9. Inhibition of JAK1-STAT3 Signaling Pathway
4.10. Rat Complete ACL Transection Model
4.11. Hematoxylin-Eosin and Masson Staining
4.12. Immunohistofluorescence Staining
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, M.; Chyr, A.; Sanders, A.P.; Raeymaekers, B. Designing prosthetic knee joints with bio-inspired bearing surfaces. Tribol. Int. 2014, 77, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.; Wang, X.Q.; Zhang, X.A. Exercise for osteoarthritis: A literature review of pathology and mechanism. Front. Aging Neurosci. 2022, 14, 854026. [Google Scholar] [CrossRef]
- Sha, Y.Q.; Zhang, B.B.; Chen, L.P.; Hong, H.H.; Chi, Q.J. Mechano Growth Factor Accelerates ACL Repair and Improves Cell Mobility of Mechanically Injured Human ACL Fibroblasts by Targeting Rac1-PAK1/2 and RhoA-ROCK1 Pathways. Int. J. Mol. Sci. 2022, 23, 4331. [Google Scholar] [CrossRef]
- Idzik, M.; Poloczek, J.; Skrzep-Poloczek, B.; Dróżdż, E.; Chełmecka, E.; Czuba, Z.; Jochem, J.; Stygar, D. The Effects of 21-Day General Rehabilitation after Hip or Knee Surgical Implantation on Plasma Levels of Selected Interleukins, VEGF, TNF-α, PDGF-BB, and Eotaxin-1. Biomolecules 2022, 12, 605. [Google Scholar] [CrossRef]
- Sha, Y.Q.; Cai, W.J.; Khalid, A.M.; Chi, Q.J.; Wang, J.; Sun, T.; Wang, C.L. Pretreatment with mechano growth factor E peptide attenuates osteoarthritis through improving cell proliferation and extracellular matrix synthesis in chondrocytes under severe hypoxia. Int. Immunopharmacol. 2021, 97, 107628. [Google Scholar] [CrossRef]
- Spindler, K.P.; Wright, R.W. Clinical practice. Anterior cruciate ligament tear. N. Engl. J. Med. 2008, 359, 2135–2142. [Google Scholar] [CrossRef] [Green Version]
- Zhen, G.H.; Guo, Q.Y.; Li, Y.S.; Wu, C.L.; Zhu, S.A.; Wang, R.M.; Guo, X.E.; Kim, B.C.; Huang, J.; Hu, Y.Z.; et al. Mechanical stress determines the configuration of TGFβ activation in articular cartilage. Nat. Commun. 2021, 12, 1706. [Google Scholar] [CrossRef]
- Liang, Q.Z.; Asila, A.; Deng, Y.J.; Liao, J.; Liu, Z.F.; Fang, R. Osteopontin-Induced lncRNA HOTAIR expression is involved in osteoarthritis by regulating cell proliferation. BMC Geriatr. 2021, 21, 57. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, S.Y. Salicin inhibits AGE-induced degradation of type II collagen and aggrecan in human SW1353 chondrocytes: Therapeutic potential in osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative stress and chronic inflammation in osteoarthritis: Can NRF2 counteract these partners in crime? Ann. N. Y. Acad. Sci. 2017, 1401, 114–135. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.C.; Wang, L.B.; Ma, C.S.; Wang, G.Z.; Zhang, Y.J.; Sun, S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019, 14, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.T.; He, C.Q. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Gao, Y.; Zhang, Z.K.; Chi, Q.J.; Liu, Y.J.; Yang, L.; Xu, K. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-κB/SIRT1/AMPK signaling pathways. Phytomedicine 2020, 78, 153305. [Google Scholar] [CrossRef]
- Yoo, K.H.; Thapa, N.; Chwae, Y.J.; Yoon, S.H.; Kim, B.J.; Lee, J.O.; Jang, Y.N.; Kim, J. Transforming growth factor-β family and stem cell-derived exosome therapeutic treatment in osteoarthritis (Review). Int. J. Mol. Med. 2022, 49, 62. [Google Scholar] [CrossRef]
- Wang, C.L.; Sha, Y.Q.; Wang, S.X.; Chi, Q.J.; Sung, K.L.P.; Xu, K.; Yang, L. Lysyl oxidase suppresses the inflammatory response in anterior cruciate ligament fibroblasts and promotes tissue regeneration by targeting myotrophin via the nuclear factor-kappa B pathway. J. Tissue Eng. Regen. Med. 2020, 14, 1063–1076. [Google Scholar] [CrossRef]
- Tan, C.N.; Zhang, Q.; Li, C.H.; Fan, J.J.; Yang, F.Q.; Hu, Y.J.; Hu, G. Potential target-related proteins in rabbit platelets treated with active monomers dehydrocorydaline and canadine from Rhizoma corydalis. Phytomedicine 2019, 54, 231–239. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, E.J.; Yoon, S.W.; Kim, C.G.; Lee, S.; Kim, J.Y.; Baek, N.; Kim, S.H. Anti-Metastatic Effect of Dehydrocorydaline on H1299 Non-Small Cell Lung Carcinoma Cells via Inhibition of Matrix Metalloproteinases and B Cell Lymphoma 2. Phytother. Res. 2017, 31, 441–448. [Google Scholar] [CrossRef]
- Hu, H.R.; Dong, Z.; Wang, X.X.; Bai, L.C.; Lei, Q.; Yang, J.; Li, L.; Li, Q.; Liu, L.C.; Zhang, Y.L.; et al. Dehydrocorydaline inhibits cell proliferation, migration and invasion via suppressing MEK1/2-ERK1/2 cascade in melanoma. Onco Targets Ther. 2019, 12, 5163–5175. [Google Scholar] [CrossRef] [Green Version]
- Huo, W.W.; Zhang, Y.; Liu, Y.; Lei, Y.S.; Sun, R.; Zhang, W.; Huang, Y.L.; Mao, Y.T.; Wang, C.C.; Ma, Z.L.; et al. Dehydrocorydaline attenuates bone cancer pain by shifting microglial M1/M2 polarization toward the M2 phenotype. Mol. Pain 2018, 14, 174486918781733. [Google Scholar] [CrossRef]
- Jin, L.S.; Zhou, S.S.; Zhu, S.J.; Lei, S.W.; Du, W.J.; Jiang, H.D.; Zeng, S.; Zhou, H. Dehydrocorydaline induced antidepressant-like effect in a chronic unpredictable mild stress mouse model via inhibiting uptake-2 monoamine transporters. Eur. J. Pharmacol. 2019, 864, 172725. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chen, I.Y.; Lee, M.Y.; Lu, C.W.; Chiu, K.M.; Wang, S.J. Inhibition of Glutamate Release from Rat Cortical Nerve Terminals by Dehydrocorydaline, an Alkaloid from Corydalis yanhusuo. Molecules 2022, 27, 960. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Li, L.; Chu, S.S.; Sun, Q.; Ma, Z.L.; Gu, X.P. Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines. Sci. Rep. 2016, 6, 27129. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.D.; Zhang, L.; Zhang, P.; Hao, Z.Y. Dehydrocorydaline Protects Against Sepsis-Induced Myocardial Injury Through Modulating the TRAF6/NF-κB Pathway. Front. Pharmacol. 2021, 12, 709604. [Google Scholar] [CrossRef]
- Najm, A.; Masson, F.M.; Preuss, P.; Georges, S.; Ory, B.; Quillard, T.; Sood, S.; Goodyear, C.S.; Veale, D.J.; Fearon, U.; et al. MicroRNA-17-5p Reduces Inflammation and Bone Erosions in Mice with Collagen-Induced Arthritis and Directly Targets the JAK/STAT Pathway in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Arthritis Rheumatol. 2020, 72, 2030–2039. [Google Scholar] [CrossRef]
- Zeng, R.; Lu, X.; Lin, J.; Ron, Z.; Fang, J.; Liu, Z.; Zeng, W. FOXM1 activates JAK1/STAT3 pathway in human osteoarthritis cartilage cell inflammatory reaction. Exp. Biol. Med. 2021, 246, 644–653. [Google Scholar] [CrossRef]
- Mohd Yunus, M.H.; Lee, Y.; Nordin, A.; Chua, K.H.; Bt Hj Idrus, R. Remodeling Osteoarthritic Articular Cartilage under Hypoxic Conditions. Int. J. Mol. Sci. 2002, 23, 5356. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Safiri, S.; Kolahi, A.A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Lauer, J.C.; Selig, M.; Hart, M.L.; Kurz, B.; Rolauffs, B. Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function. Int. J. Mol. Sci. 2021, 22, 3279. [Google Scholar] [CrossRef]
- Sakalyte, R.; Denkovskij, J.; Bernotiene, E.; Stropuviene, S.; Mikulenaite, S.O.; Kvederas, G.; Porvaneckas, N.; Tutkus, V.; Venalis, A.; Butrimiene, I. The Expression of Inflammasomes NLRP1 and NLRP3, Toll-Like Receptors, and Vitamin D Receptor in Synovial Fibroblasts From Patients With Different Types of Knee Arthritis. Front. Immunol. 2022, 12, 767512. [Google Scholar] [CrossRef] [PubMed]
- Drummer, D.J.; McAdam, J.S.; Seay, R.; Aban, I.; Lavin, K.M.; Wiggins, D.; Touliatos, G.; Yang, S.; Kelley, C.; Tuggle, S.C.; et al. Perioperative assessment of muscle inflammation susceptibility in patients with end-stage osteoarthritis. J. Appl. Physiol. 2022, 132, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Chan, P.; Wen, C. Do immune cells lead the way in subchondral bone disturbance in osteoarthritis? Prog. Biophys. Mol. Biol. 2019, 148, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, J.; Wu, J.; Chen, Q.; Du, W.; Fu, F.; Yu, H.; Yao, S.; Jin, H.; Tong, P.; et al. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J. Ethnopharmacol. 2020, 247, 112261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, Y.; Sun, X.; Xing, Y.; Wang, X.; Yang, Q. Immunomodulation of MSCs and MSC-Derived Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2020, 8, 575057. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Koelling, S.; Kruegel, J.; Irmer, M.; Path, J.R.; Sadowski, B.; Miro, X.; Miosge, N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009, 4, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Gerter, R.; Kruegel, J.; Miosge, N. New insights into cartilage repair—The role of migratory progenitor cells in osteoarthritis. Matrix Biol. 2012, 31, 206–213. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Liu, X.; Li, L.; Xu, H.; Dong, N.; Xu, K. Andrographolide ameliorates aortic valve calcification by regulation of lipid biosynthesis and glycerolipid metabolism targeting MGLL expression in vitro and in vivo. Cell Calcium 2021, 100, 102495. [Google Scholar] [CrossRef]
- Lv, Y.; Hao, X.; Sha, Y.; Yang, L. Pretreatment with mechano-growth factor E peptide protects bone marrow mesenchymal cells against damage by fluid shear stress. Biotechnol. Lett. 2014, 36, 2559–2569. [Google Scholar] [CrossRef]
- Sha, Y.Q.; Afandi, R.; Zhang, B.B.; Yang, L.; Lv, Y.G. MGF E peptide pretreatment improves collagen synthesis and cell proliferation of injured human ACL fibroblasts via MEK-ERK1/2 signaling pathway. Growth Factors 2017, 35, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, Y.; Qu, L.; Liu, Y.; Liu, X.; Xu, K. Cardamonin inhibits osteogenic differentiation of human valve interstitial cells and ameliorates aortic valve calcification viainterfering in the NF-κB/NLRP3 inflammasome pathway. Food Funct. 2021, 12, 11808–11818. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.Q.; Yang, L.; Lv, Y.G. MGF E peptide improves anterior cruciate ligament repair by inhibiting hypoxia-induced cell apoptosis and accelerating angiogenesis. J. Cell. Physiol. 2019, 234, 8846–8861. [Google Scholar] [CrossRef]







| Gene. | Primer Sequence | Amplicon Length |
|---|---|---|
| Aggrecan | F: 5′- ACTCTGGGTTTTCGTGACTCT -3′ | 81 bp |
| R: 5′- ACACTCAGCGAGTTGTCATGG -3′ | ||
| Collagen 1 | F: 5′- CTGGAAGAGTGGAGAGTACTG -3′ | 143 bp |
| R: 5′- TGCTGATGTACCAGTTCTTCTG -3′ | ||
| Collagen 2 | F: 5′- CCAGATGACCTTCCTACGCC -3′ | 186 bp |
| R: 5′- TTCAGGGCAGTGTACGTGAAC -3′ | ||
| MMP1 | F: 5′- AAAATTACACGCCAGATTTGCC -3′ | 82 bp |
| R: 5′- GGTGTGACATTACTCCAGAGTTG -3′ | ||
| MMP13 | F: 5′- CCAGACTTCACGATGGCATTG -3′ | 137 bp |
| R: 5′- GGCATCTCCTCCATAATTTGGC -3′ | ||
| Cox2 | F: 5′- ATGCTGACTATGGCTACAAAAGC -3′ | 90 bp |
| R: 5′- TCGGGCAATCATCAGGCAC -3′ | ||
| Jak1 | F: 5′- CCACTACCGGATGAGGTTCTA -3′ | 213 bp |
| R: 5′- GGGTCTCGAATAGGAGCCAG -3′ | ||
| STAT3 | F: 5′- ACCAGCAGTATAGCCGCTTC -3′ | 124 bp |
| R: 5′- GCCACAATCCGGGCAATCT -3′ | ||
| GAPDH | F: 5′- GGATTTGGTCGTATTGGG -3′ | 218 bp |
| R: 5′- GCTCCTGGAAGATGGTGAT -3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, Y.; Zhang, B.; Chen, L.; Wang, C.; Sun, T. Dehydrocorydaline Accelerates Cell Proliferation and Extracellular Matrix Synthesis of TNFα-Treated Human Chondrocytes by Targeting Cox2 through JAK1-STAT3 Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 7268. https://doi.org/10.3390/ijms23137268
Sha Y, Zhang B, Chen L, Wang C, Sun T. Dehydrocorydaline Accelerates Cell Proliferation and Extracellular Matrix Synthesis of TNFα-Treated Human Chondrocytes by Targeting Cox2 through JAK1-STAT3 Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(13):7268. https://doi.org/10.3390/ijms23137268
Chicago/Turabian StyleSha, Yongqiang, Beibei Zhang, Liping Chen, Chunli Wang, and Tao Sun. 2022. "Dehydrocorydaline Accelerates Cell Proliferation and Extracellular Matrix Synthesis of TNFα-Treated Human Chondrocytes by Targeting Cox2 through JAK1-STAT3 Signaling Pathway" International Journal of Molecular Sciences 23, no. 13: 7268. https://doi.org/10.3390/ijms23137268